Primer and platform effects on 16S rRNA tag sequencing
- PMID: 26300854
- PMCID: PMC4523815
- DOI: 10.3389/fmicb.2015.00771
Primer and platform effects on 16S rRNA tag sequencing
Abstract
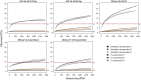
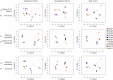
Sequencing of 16S rRNA gene tags is a popular method for profiling and comparing microbial communities. The protocols and methods used, however, vary considerably with regard to amplification primers, sequencing primers, sequencing technologies; as well as quality filtering and clustering. How results are affected by these choices, and whether data produced with different protocols can be meaningfully compared, is often unknown. Here we compare results obtained using three different amplification primer sets (targeting V4, V6-V8, and V7-V8) and two sequencing technologies (454 pyrosequencing and Illumina MiSeq) using DNA from a mock community containing a known number of species as well as complex environmental samples whose PCR-independent profiles were estimated using shotgun sequencing. We find that paired-end MiSeq reads produce higher quality data and enabled the use of more aggressive quality control parameters over 454, resulting in a higher retention rate of high quality reads for downstream data analysis. While primer choice considerably influences quantitative abundance estimations, sequencing platform has relatively minor effects when matched primers are used. Beta diversity metrics are surprisingly robust to both primer and sequencing platform biases.
Keywords: 16S rRNA gene sequencing; amplification; community assembly; high throughput sequencing; microbial diversity; microbial population and community ecology; sequencing error.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

