Pseudomonas aeruginosa PAO-1 Lipopolysaccharide-Diphtheria Toxoid Conjugate Vaccine: Preparation, Characterization and Immunogenicity
- PMID: 26301059
- PMCID: PMC4541022
- DOI: 10.5812/jjm.8(5)2015.17712
Pseudomonas aeruginosa PAO-1 Lipopolysaccharide-Diphtheria Toxoid Conjugate Vaccine: Preparation, Characterization and Immunogenicity
Abstract
Background: Treatment of Pseudomonas aeruginosa PAO-1 infections through immunological means has been proved to be efficient and protective.
Objectives: The purpose of this study was to produce a conjugate vaccine composed of detoxified lipopolysaccharide (D-LPS) P. aeruginosa and diphtheria toxoid (DT).
Materials and methods: Firstly, LPS was purified and characterized from P. aeruginosa PAO1 and then detoxified. D-LPS was covalently coupled to DT as a carrier protein via amidation method with adipic acid dihydrazide (ADH) as a spacer molecule and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (EDAC) as a linker. The molar ratio of LPS to DT in the prepared conjugate was 3:1. The immunogenicity of D-LPS-DT conjugate vaccine in mice model was evaluated as well.
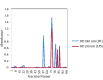
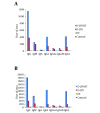
Results: The conjugate was devoid of endotoxin activity and 0.125 U/mL of D-LPS was acceptable for immunization. D-LPS-DT conjugate was nonpyrogenic for rabbits and nontoxic for mice. Mice immunization with D-LPS-DT conjugate vaccine elicited the fourfold higher IgG antibody compared to D-LPS. Anti-LPS IgG antibody was predominantly IgG1 subclass and then IgG3, IgG2a and IgG2b, respectively.
Conclusions: Vaccine based on the conjugation of P. aeruginosa PAO-1 LPS with DT increased anti-LPS antibodies and had a significant potential to protect against Pseudomonas infections.
Keywords: Diphtheria Toxoid; Lipopolysaccharides; Pseudomonas aeruginosa PAO-1; Vaccines, Conjugate.
Figures

Similar articles
-
Immunogenicity comparison of conjugate vaccines composed of alginate and lipopolysaccharide of Pseudomonas aeruginosa bound to diphtheria toxoid.Iran J Microbiol. 2014 Oct;6(5):317-23. Iran J Microbiol. 2014. PMID: 25848521 Free PMC article.
-
Synthesis, characterization and immunological properties of Escherichia coli 0157:H7 lipopolysaccharide- diphtheria toxoid conjugate vaccine.Iran J Microbiol. 2015 Jun;7(3):150-5. Iran J Microbiol. 2015. PMID: 26668702 Free PMC article.
-
Pseudomonas aeruginosa polysaccharide-tetanus toxoid conjugate vaccine: safety and immunogenicity in humans.J Infect Dis. 1986 Oct;154(4):682-8. doi: 10.1093/infdis/154.4.682. J Infect Dis. 1986. PMID: 3091708
-
Effect of immunity to the carrier protein on antibody responses to Haemophilus influenzae type b conjugate vaccines.Vaccine. 1993;11 Suppl 1:S46-51. doi: 10.1016/0264-410x(93)90160-y. Vaccine. 1993. PMID: 8447176 Review.
-
Pseudomonas aeruginosa antigens as potential vaccines.FEMS Microbiol Rev. 1997 Nov;21(3):243-77. doi: 10.1111/j.1574-6976.1997.tb00353.x. FEMS Microbiol Rev. 1997. PMID: 9451816 Review.
Cited by
-
Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa.Drug Des Devel Ther. 2019 Mar 20;13:909-924. doi: 10.2147/DDDT.S189847. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30936684 Free PMC article. Review.
-
Characterization of Toxin-Antitoxin (TA) Systems in Pseudomonas aeruginosa Clinical Isolates in Iran.Jundishapur J Microbiol. 2016 Jan 2;9(1):e26627. doi: 10.5812/jjm.26627. eCollection 2016 Jan. Jundishapur J Microbiol. 2016. PMID: 27099681 Free PMC article.
-
Immunization against Pseudomonas aeruginosa using Alg-PLGA nano-vaccine.Iran J Basic Med Sci. 2021 Apr;24(4):476-482. doi: 10.22038/ijbms.2021.52217.11813. Iran J Basic Med Sci. 2021. PMID: 34094029 Free PMC article.
-
Evaluation of the Immunogenicity of Diphtheria Toxoid Conjugated to Salmonella Typhimurium-Derived OPS in a Mouse Model: A Potential Vaccine Candidate Against Salmonellosis.Iran Red Crescent Med J. 2016 May 30;18(7):e34135. doi: 10.5812/ircmj.34135. eCollection 2016 Jul. Iran Red Crescent Med J. 2016. PMID: 27660722 Free PMC article.
-
Understanding Pseudomonas aeruginosa-Host Interactions: The Ongoing Quest for an Efficacious Vaccine.Cells. 2020 Dec 5;9(12):2617. doi: 10.3390/cells9122617. Cells. 2020. PMID: 33291484 Free PMC article. Review.
References
-
- Siegel RE. Emerging gram-negative antibiotic resistance: daunting challenges, declining sensitivities, and dire consequences. Respir Care. 2008;53(4):471–9. - PubMed
-
- Taheri ZM, Shahbazi N, Khoddami M. Genetic Diversity of pseudomonas aeruginosa Strains isolated from Hospitalized patients. Tanaffos. 2008;7(1):32–9.
-
- Al-Zeer M, Masoud H. LPS-based conjugate vaccines composed of O-polysaccharide from Pseudomonas aeruginosa IATS 6 and 11 bound to a carrier protein. World J Microbiol Biotechnol . 2007;23(11):1541–9.
-
- Donta ST, Peduzzi P, Cross AS, Sadoff J, Haakenson C, Cryz SJ, et al. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa Infections. J Infect Dis. 1996;174(3):537–43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
