The Omega-3 Fatty Acid Docosahexaenoic Acid Modulates Inflammatory Mediator Release in Human Alveolar Cells Exposed to Bronchoalveolar Lavage Fluid of ARDS Patients
- PMID: 26301250
- PMCID: PMC4537738
- DOI: 10.1155/2015/642520
The Omega-3 Fatty Acid Docosahexaenoic Acid Modulates Inflammatory Mediator Release in Human Alveolar Cells Exposed to Bronchoalveolar Lavage Fluid of ARDS Patients
Abstract
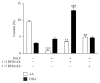
Background: This study investigated whether the 1 : 2 ω-3/ω-6 ratio may reduce proinflammatory response in human alveolar cells (A549) exposed to an ex vivo inflammatory stimulus (bronchoalveolar lavage fluid (BALF) of acute respiratory distress syndrome (ARDS) patients). Methods. We exposed A549 cells to the BALF collected from 12 ARDS patients. After 18 hours, fatty acids (FA) were added as docosahexaenoic acid (DHA, ω-3) and arachidonic acid (AA, ω-6) in two ratios (1 : 2 or 1 : 7). 24 hours later, in culture supernatants were evaluated cytokines (TNF-α, IL-6, IL-8, and IL-10) and prostaglandins (PGE2 and PGE3) release. The FA percentage content in A549 membrane phospholipids, content of COX-2, level of PPARγ, and NF-κB binding activity were determined.
Results: The 1 : 2 DHA/AA ratio reversed the baseline predominance of ω-6 over ω-3 in the cell membranes (P < 0.001). The proinflammatory cytokine release was reduced by the 1 : 2 ratio (P < 0.01 to <0.001) but was increased by the 1 : 7 ratio (P < 0.01). The 1 : 2 ratio reduced COX-2 and PGE2 (P < 0.001) as well as NF-κB translocation into the nucleus (P < 0.01), while it increased activation of PPARγ and IL-10 release (P < 0.001). Conclusion. This study demonstrated that shifting the FA supply from ω-6 to ω-3 decreased proinflammatory mediator release in human alveolar cells exposed to BALF of ARDS patients.
Figures






Similar articles
-
Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells.Lipids Health Dis. 2013 Jul 13;12:101. doi: 10.1186/1476-511X-12-101. Lipids Health Dis. 2013. PMID: 23849180 Free PMC article.
-
Emodin improves alveolar hypercoagulation and inhibits pulmonary inflammation in LPS-provoked ARDS in mice via NF-κB inactivation.Int Immunopharmacol. 2020 Nov;88:107020. doi: 10.1016/j.intimp.2020.107020. Epub 2020 Sep 21. Int Immunopharmacol. 2020. PMID: 33182048
-
ω-3 PUFAs and Resveratrol Differently Modulate Acute and Chronic Inflammatory Processes.Biomed Res Int. 2015;2015:535189. doi: 10.1155/2015/535189. Epub 2015 Aug 2. Biomed Res Int. 2015. PMID: 26301248 Free PMC article.
-
Omega-6 fatty acids and inflammation.Prostaglandins Leukot Essent Fatty Acids. 2018 May;132:41-48. doi: 10.1016/j.plefa.2018.03.004. Epub 2018 Mar 22. Prostaglandins Leukot Essent Fatty Acids. 2018. PMID: 29610056 Review.
-
Immunonutrition for acute respiratory distress syndrome (ARDS) in adults.Cochrane Database Syst Rev. 2019 Jan 24;1(1):CD012041. doi: 10.1002/14651858.CD012041.pub2. Cochrane Database Syst Rev. 2019. PMID: 30677127 Free PMC article.
Cited by
-
Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer.Biochimie. 2021 Aug;187:94-109. doi: 10.1016/j.biochi.2021.05.013. Epub 2021 May 31. Biochimie. 2021. PMID: 34082041 Free PMC article.
-
Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS).J Clin Med. 2022 Dec 30;12(1):304. doi: 10.3390/jcm12010304. J Clin Med. 2022. PMID: 36615103 Free PMC article. Review.
-
Associations between fatty acids and low-grade inflammation in children from the LISAplus birth cohort study.Eur J Clin Nutr. 2017 Nov;71(11):1303-1311. doi: 10.1038/ejcn.2017.73. Epub 2017 Jun 7. Eur J Clin Nutr. 2017. PMID: 28589948
-
Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer.Antioxidants (Basel). 2021 Oct 29;10(11):1734. doi: 10.3390/antiox10111734. Antioxidants (Basel). 2021. PMID: 34829605 Free PMC article. Review.
-
A high docosahexaenoic acid diet alters lung inflammation and recovery following repetitive exposure to aqueous organic dust extracts.J Nutr Biochem. 2021 Nov;97:108797. doi: 10.1016/j.jnutbio.2021.108797. Epub 2021 Jun 12. J Nutr Biochem. 2021. PMID: 34126202 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

