Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma
- PMID: 26301488
- PMCID: PMC8666145
- DOI: 10.1002/14651858.CD011397.pub2
Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma
Abstract
Background: Despite the availability of several evidence-based therapies and non-pharmacological strategies to improve control of symptoms and prevent exacerbations of asthma, patients with asthma continue to be at risk for mortality and morbidity.Previous trials have demonstrated the potentially beneficial effects of the long-acting muscarinic antagonist (LAMA) tiotropium on lung function in patients with asthma; however, a definitive conclusion on the benefit of LAMA in asthma is lacking, as is information on where in the current step-wise management strategy they would be most beneficial.
Objectives: To assess the efficacy and safety of a LAMA added to any dose of an inhaled corticosteroid (ICS) compared with the same dose of ICS alone for adults whose asthma is not well controlled.
Search methods: We searched the Cochrane Airways Group Specialised Register (CAGR) from inception to April 2015, and we imposed no restriction on language of publication. We also searched clinicaltrials.gov, the World Health Organization (WHO) trials portal and drug company registries to identify unpublished studies.
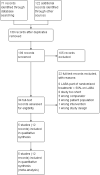
Selection criteria: We searched for parallel and cross-over randomised controlled trials in which adults whose asthma was not well controlled by ICS alone were randomly assigned to receive LAMA add-on or placebo (both combined with ICS) for at least 12 weeks.
Data collection and analysis: Two review authors independently screened the searches and extracted data from study reports. We used Covidence for duplicate screening, extraction of study characteristics and numerical data and risk of bias ratings. Pre-specified primary outcomes included exacerbations requiring oral corticosteroids, quality of life and all-cause serious adverse events.
Main results: We identified five studies that met the inclusion criteria. All studies applied a double-blind, double-dummy design, and the population of all studies totalled 2563 adult participants. Study duration ranged from 12 weeks to 52 weeks, and risk of bias across domains in all studies was low. Trials included more women than men (33% to 47% male), and mean age of participants ranged from 41 to 48 years. Participants generally had a long history of asthma, and mean baseline predicted forced expiratory volume in one second (FEV1) was between 72% and 75% in three studies reporting pre-bronchodilator values.The rate of exacerbations requiring oral corticosteroids (OCS) was lower in patients prescribed an LAMA add-on than in those receiving the same dose of ICS alone (odds ratio (OR) 0.65, 95% confidence interval (CI) 0.46 to 0.93; 2277 participants; four studies; I(2) = 0%; high-quality evidence), meaning that 27 fewer people per 1000 would have an exacerbation over 21 weeks requiring OCS with LAMA compared with ICS alone (95% CI 42 fewer to 6 fewer).All-cause serious adverse events (SAEs) and exacerbations requiring hospital admission were rare and the effects too imprecise to permit firm conclusions, but effects suggested that LAMA add-on may be associated with fewer of both compared with ICS alone (SAEs: OR 0.60, 95% CI 0.23 to 1.57; 2532 participants; four studies; low-quality evidence; exacerbations requiring hospital admission: OR 0.42, 95% CI 0.12 to 1.47; 2562 participants; five studies; moderate-quality evidence). Additional therapy with a LAMA showed no clear benefit in terms of quality of life compared with ICS given alone; high-quality evidence showed only a small mean improvement in quality of life as measured on the Asthma Quality of Life Questionnaire (AQLQ), which was not statistically significant. The same was true for asthma control as measured on the Asthma Control Questionnaire (ACQ), which was based on moderate-quality evidence. LAMA combined with ICS showed consistent benefit in a range of lung function measures compared with the same dose of ICS alone, and LAMA was not associated with significantly higher rates of adverse events than were reported with placebo.
Authors' conclusions: For adults taking ICS for asthma without a long-acting beta₂-agonist (LABA), LAMA given as add-on treatment reduces the likelihood of exacerbations requiring treatment with OCS and improves lung function. The benefits of LAMA combined with ICS for hospital admissions, all-cause serious adverse events, quality of life and asthma control remain unknown.Results of this review, along with findings of related reviews conducted to assess the use of LAMA in other clinical scenarios involving asthma, can help to define the role of LAMA in the management of asthma. Trials of longer duration (up to 52 weeks) would provide a better opportunity to observe rare events such as serious adverse events and exacerbations requiring hospital admission.
Conflict of interest statement
Debbie Allison: none known.
Kayleigh Kew: none known.
Anne Boyter: none known.
Figures






















Update of
- doi: 10.1002/14651858.CD011397
References
References to studies included in this review
NCT00350207 {published and unpublished data}
-
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is non inferior to salmeterol in maintaining improved lung function in B16‐Arg/Arg patients with asthma. Journal of Allergy and Clinical Immunology 2011;128(2):315‐22. - PubMed
-
- Dahl R, Bateman ED, Casale T, Pizzichini E, Vandewalker M, Virchow JC, et al. Once‐daily tiotropium Respimat as add‐on to at least medium‐ to high‐dose ICS, with or without LABA, improves lung function in patients with symptomatic asthma, independent of allergic status. 33rd Congress of the European Academy of Allergy and Clinical Immunology, Copenhagen. 2014:515.
-
- NCT00350207. A 16‐week randomised, placebo‐controlled, double‐blind, double‐dummy, parallel‐group study comparing the efficacy and safety of tiotropium inhalation solution delivered by the Respimat inhaler (2 actuations of 2.5 mcg once daily) with that of salmeterol from the hydrofluoroalkane metered dose inhaler (2 actuations of 25 mcg twice daily) in moderate persistent asthma patients with the B16‐Arg/Arg genotype. http://www.clinicaltrials.gov/show/NCT00350207 (accessed 19 December 2014).
NCT01172808 {published and unpublished data}
-
- Kerstjens HAM, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add‐on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double‐blind, placebo‐controlled,parallel‐group, active‐comparator, randomised trials. Lancet Respiratory Medicine 2015 Feb 12 [Epub ahead of print]. - PubMed
-
- NCT01172808. A Phase III Randomised, Double‐blind, Placebo‐controlled, Parallel‐group Trial to Evaluate Efficacy and Safety of Tiotropium Inhalation Solution Delivered Via Respimat® Inhaler (2.5 and 5 mcg Once Daily) Compared With Placebo and Salmeterol HFA MDI (50 mcg Twice Daily) Over 24 Weeks in Moderate Persistent Asthma. http://www.clinicaltrials.gov/show/NCT01172808 (accessed 19 December 2014).
NCT01172821 {published and unpublished data}
-
- Casale T, Bleecker E, Meltzer E, Pizzichini E, Schmidt O, Bateman E, et al. Phase III trials to investigate tiotropium as add‐on therapy to inhaled corticosteroids for patients with symptomatic asthma: trial design and planned statistical analyses. Allergy 2013;68:377.
-
- Casale TB, Bateman ED, Dahl R, Pizzichini E, Vandewalker ML, Virchow JC, et al. Tiotropium Respimat add‐on therapy reduces airflow obstruction in patients with symptomatic moderate asthma, independent of Th2 inflammatory status. Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB5.
-
- Kerstjens HAM, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add‐on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double‐blind, placebo‐controlled, parallel‐group, active‐comparator, randomised trials. Lancet Respiratory Medicine 2015 Feb 12 [Epub ahead of print]. - PubMed
-
- NCT01172821. A Phase III Randomised, Double‐blind, Placebo‐controlled, Parallel‐group Trial to Evaluate Efficacy and Safety of Tiotropium Inhalation Solution Delivered Via Respimat® Inhaler (2.5 and 5 mcg Once Daily) Compared With Placebo and Salmeterol HFA MDI (50 mcg Twice Daily) Over 24 Weeks in Moderate Persistent Asthma. http://www.clinicaltrials.gov/show/NCT01172821 (accessed 19 December 2014).
NCT01316380 {published and unpublished data}
-
- NCT01316380. A phase III, randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (2.5 mcg and 5 mcg once daily) compared to placebo over 12 weeks in mild persistent asthma. http://www.clinicaltrials.gov/show/NCT01316380 (accessed 19 December 2014).
-
- Paggiaro P, Engel M, Tudoric N, Forstner B, Radeczky E, Zubek V, et al. Phase III trial of tiotropium as add‐on therapy to low‐dose inhaled corticosteroids for patients with symptomatic mild persistent asthma: design and planned analyses [Abstract]. European Respiratory Journal 2013;42(Suppl 57):877s [P4133].
-
- Paggiaro P, Halpin DMG, Buhl R, Engel M, Zubek V, Blahova Z, et al. Tiotropium Respimat add‐on to inhaled corticosteroids improves lung function in patients with symptomatic mild asthma: results from a phase III trial. Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB4.
NCT01340209 {published and unpublished data}
-
- NCT01340209. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate safety and efficacy of tiotropium inhalation solution delivered via Respimat inhaler (2.5 and 5 µg once daily) compared with placebo over 52 weeks in patients with moderate to severe persistent asthma. http://www.clinicaltrials.gov/show/NCT01340209 (accessed 19 December 2014).
-
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Long‐term once daily tiotropium Respimat is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo‐controlled study. PLoS ONE 2015;10(4):e0124109. - PMC - PubMed
References to studies excluded from this review
CTRI/2008/091/000306 {published data only}
-
- Chest Research Foundation. A clinical trial comparing bronchodilator effects of two drugs, tiotropium bromide and formoterol fumarate, each over 24 hours in subjects with asthma. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2008/091/000306 (accessed 19 December 2014).
CTRI/2012/08/002915 {published data only}
-
- Ilango Dr K. A clinical trial to study the effects of few anti asthmatic drug combinations on asthma patients. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/08/002915 (accessed 19 December 2014).
EUCTR2006‐003385‐34‐NL {published data only}
-
- Boehringer Ingelheim Pharma GmbH, Co. KG. The effect of tiotropium‐bromide on deep inspiration‐induced bronchodilation and airway responsiveness in asthma ‐ Tiotropium‐bromide and lung mechanics. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006‐003385‐34‐NL (accessed 19 December 2014).
JPRN‐UMIN000003618 {published data only}
-
- Saitama Medical University. Randomized clinical study with tiotropium and formoterol/budesonide in COPD with asthma. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000003618 (accessed 19 December 2014).
JPRN‐UMIN000005459 {published data only}
-
- EACC (Ehime Asthma COPD Conference). Add‐on therapy for COPD receiving inhaled bronchodilators. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000005459 (accessed 19 December 2014).
JPRN‐UMIN000010352 {published data only}
-
- Fukuoka National Hospital. Study for effects of tiotropium on smokers and non‐smokers with bronchial asthma. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000010352 (accessed 19 December 2014).
Kerstjens 2012 {published data only}
-
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians 2013;144:91A.
-
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198‐207. - PubMed
-
- Vandewalker ML, Engel M, Schmidt H, Siebold W, Moroni‐Zentgraf P, Kerstjens H, et al. Efficacy of tiotropium in patients with asthma in relation to allergic status [Abstract]. Journal of Allergy and Clinical Immunology 2013;131(2 Suppl 1):AB1.
NCT00546234 {published data only}
-
- NCT00546234. Assessing treatment options for smokers with asthma. www.clinicaltrials.gov/show/NCT00546234 (accessed 4 March 2015).
NCT00557180 {published data only}
-
- National Jewish Health. Examining the link between obesity, inflammation, and response to asthma medications. https://clinicaltrials.gov/show/NCT00557180 (accessed 19 December 2014).
NCT00557700 {published data only}
-
- NCT00557700. Study to investigate the effect of inhaled tiotropium bromide on neurokinin‐A induced bronchoconstriction in patients with mild‐to‐moderate asthma. www.clinicaltrials.gov/show/NCT00557700 (accessed 4 March 2015).
NCT00706446 {published data only}
-
- NCT00706446. Genotype stratified treatment with anticholinergic vs. beta‐agonist (long‐acting) and exacerbations (GABLE). www.clinicaltrials.gov/show/NCT00706446 (accessed 4 March 2015).
NCT00772538 {published data only}
-
- NCT00772538. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (5 mcg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. www.clinicaltrials.gov/show/NCT00772538 (accessed 4 March 2015).
NCT00776984 {published data only}
-
- NCT00776984. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (5 mcg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. www.clinicaltrials.gov/show/NCT00776984 (accessed 4 March 2015).
NCT01290874 {published data only}
-
- NCT01290874. Blacks and exacerbations on LABA vs. tiotropium (BELT). www.clinicaltrials.gov/show/NCT01290874 (accessed 4 March 2015).
NCT01573624 {published data only}
-
- NCT01573624. A multi‐center, randomized, double‐blind, dose‐ranging study to evaluate GSK573719 in combination with fluticasone furoate, fluticasone furoate alone, and an active control of fluticasone furoate/vilanterol combination in subjects with asthma. www.clinicaltrials.gov/show/NCT01573624 (accessed 4 March 2015).
NCT01641692 {published data only}
-
- NCT01641692. A multi‐national, randomized, double‐blind, placebo‐controlled, 3‐period crossover study with GSK 573719 as monotherapy in adult subjects with asthma. www.clinicaltrials.gov/show/NCT01641692 (accessed 4 March 2015).
NCT01696214 {published data only}
-
- NCT01696214. SAPS: Smoking Asthmatics Pilot Study. www.clinicaltrials.gov/show/NCT01696214 (accessed 4 March 2015).
NCT02066298 {published data only}
-
- NCT02066298. Steroids in eosinophil negative asthma. www.clinicaltrials.gov/show/NCT02066298 (accessed 4 March 2015).
NCT02127697 {published data only}
-
- NCT02127697. A randomized, double‐blind, parallel group, 52‐week study evaluating the efficacy, safety and tolerability of NVA237 in patients with poorly controlled asthma. www.clinicaltrials.gov/show/NCT02127697 (accessed 4 March 2015).
Vogelberg 2014 {published data only}
-
- Vogelberg C, Engel M, Moroni‐Zentgraf P, Leonaviciute‐Klimantaviciene M, Sigmund R, Downie J, et al. Once‐daily tiotropium in adolescents with symptomatic asthma despite inhaled corticosteroid treatment: a dose‐ranging study [Abstract]. Clinical and Translational Allergy 2014;4(Suppl 1):2 [O5].
Additional references
Asthma UK 2014
-
- Asthma UK. Asthma Facts and FAQs. http://www.asthma.org.uk/asthma‐facts‐and‐statistics (accessed 20 August 2014).
Barnes 1996
Beeh 2014
Befekadu 2014
Brozek 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
BTS/SIGN 2012
-
- British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN). British Guideline on the Management of Asthma. https://www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/ (accessed 29 July 2014).
CDC 2014
-
- Centers for Disease Control and Prevention. Asthma Surveillance Data. http://www.cdc.gov/asthma/ (accessed 20 August 2014).
Chauhan 2013
Chauhan 2014
Covidence [Computer program]
-
- The Alfred Hospital, Monash University, National ICT Australia and the University of London. Covidence. Prahran VIC: Alfred Health, 2013.
DOH 2012
-
- Department of Health. An outcomes strategy for COPD and asthma: NHS companion document. https://www.gov.uk/government/publications/an‐outcomes‐strategy‐for‐copd... (accessed 20 August 2014).
Ducharme 2010
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] - DOI - PMC - PubMed
EMC 2013a
-
- Boehringer Ingelheim Limited. Summary of product characteristics: Spiriva 18 microgram inhalation powder, hard capsule. https://www.medicines.org.uk/emc/medicine/10039 (accessed 20 August 2014).
EMC 2013b
-
- Boehringer Ingelheim Limited. Summary of product characteristics: Spiriva Respimat 2.5 micrograms solution for inhalation. https://www.medicines.org.uk/emc/medicine/20134 (accessed 20 August 2014).
eMC 2014a
-
- Electronic Medicines Compendium. License extension 19th September 2014: Spiriva Respimat 2.5 micrograms solution for inhalation. https://www.medicines.org.uk/emc/history/20134 (accessed 24 October 2014).
EMC 2014b
-
- Almirall Limited. Sumary of product characteristics: Eklira Genuair 322 micrograms inhalation powder. https://www.medicines.org.uk/emc/medicine/27001 (accessed 20 August 2014).
GINA 2014a
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf (accessed 28 July 2014).
GINA 2014b
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention—Revised 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf (accessed 20 October 2014). [http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf]
Global Asthma Report 2011
-
- International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. http://www.theunion.org/what‐we‐do/publications/english/global_asthma_re... (accessed 4 August 2014).
Global Burden of Asthma Report 2004
-
- Masoli M, Fabian D, Holt S, Beasley R. Global Burden of Asthma. http://www.ginasthma.org/local/uploads/files/GINABurdenReport_1.pdf (accessed 16 September 2014).
Gosens 2006
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Karner 2014
Kew 2015
-
- Kew KM, Evans DJW, Anderson DE, Boyter AC. Long‐acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long‐acting beta2‐agonists (LABA) for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011438.pub2] - DOI - PMC - PubMed
NICE 2010
-
- National Institute of Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care CG101. http://www.nice.org.uk/guidance/CG101 (accessed 20 August 2014).
Peters 2010
Review Manager 2014 (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigo 2015
Tian 2014
Timmer 2014
WHO 2007
-
- World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: A comprehensive approach. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Geneva, Switzerland: World Health Organization, 2007.

