Boron bridging of rhamnogalacturonan-II is promoted in vitro by cationic chaperones, including polyhistidine and wall glycoproteins
- PMID: 26301520
- PMCID: PMC4973674
- DOI: 10.1111/nph.13596
Boron bridging of rhamnogalacturonan-II is promoted in vitro by cationic chaperones, including polyhistidine and wall glycoproteins
Abstract
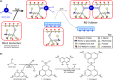
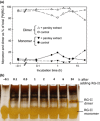
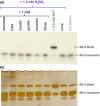
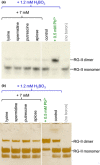
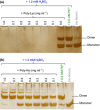
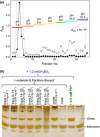
Dimerization of rhamnogalacturonan-II (RG-II) via boron cross-links contributes to the assembly and biophysical properties of the cell wall. Pure RG-II is efficiently dimerized by boric acid (B(OH)3 ) in vitro only if nonbiological agents for example Pb(2+) are added. By contrast, newly synthesized RG-II domains dimerize very rapidly in vivo. We investigated biological agents that might enable this. We tested for three such agents: novel enzymes, borate-transferring ligands and cationic 'chaperones' that facilitate the close approach of two polyanionic RG-II molecules. Dimerization was monitored electrophoretically. Parsley shoot cell-wall enzymes did not affect RG-II dimerization in vitro. Borate-binding ligands (apiose, dehydroascorbic acid, alditols) and small organic cations (including polyamines) also lacked consistent effects. Polylysine bound permanently to RG-II, precluding electrophoretic analysis. However, another polycation, polyhistidine, strongly promoted RG-II dimerization by B(OH)3 without irreversible polyhistidine-RG-II complexation. Likewise, partially purified spinach extensins (histidine/lysine-rich cationic glycoproteins), strongly promoted RG-II dimerization by B(OH)3 in vitro. Thus certain polycations, including polyhistidine and wall glycoproteins, can chaperone RG-II, manoeuvring this polyanionic polysaccharide domain such that boron-bridging is favoured. These chaperones dissociate from RG-II after facilitating its dimerization, indicating that they act catalytically rather than stoichiometrically. We propose a natural role for extensin-RG-II interaction in steering cell-wall assembly.
Keywords: Boron; cell wall; cross-linking; dehydroascorbic acid; extension; pectic polysaccharides; polyhistidine; rhamnogalacturonan-II (RG-II).
© 2015 The Authors. New Phytologist © 2015 New Phytologist Trust.
Figures


Similar articles
-
Making and breaking of boron bridges in the pectic domain rhamnogalacturonan-II at apoplastic pH in vivo and in vitro.Plant J. 2023 Mar;113(6):1310-1329. doi: 10.1111/tpj.16112. Epub 2023 Feb 8. Plant J. 2023. PMID: 36658763 Free PMC article.
-
Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion.Plant J. 2014 Feb;77(4):534-46. doi: 10.1111/tpj.12403. Epub 2014 Jan 24. Plant J. 2014. PMID: 24320597 Free PMC article.
-
An Arabidopsis thaliana arabinogalactan-protein (AGP31) and several cationic AGP fragments catalyse the boron bridging of rhamnogalacturonan-II.Biochem J. 2022 Sep 30;479(18):1967-1984. doi: 10.1042/BCJ20220340. Biochem J. 2022. PMID: 36062804 Free PMC article.
-
Arabinogalactan-Proteins as Boron-Acting Enzymes, Cross-Linking the Rhamnogalacturonan-II Domains of Pectin.Plants (Basel). 2023 Nov 21;12(23):3921. doi: 10.3390/plants12233921. Plants (Basel). 2023. PMID: 38068557 Free PMC article. Review.
-
Regulation, Diversity and Evolution of Boron Transporters in Plants.Plant Cell Physiol. 2021 Sep 24;62(4):590-599. doi: 10.1093/pcp/pcab025. Plant Cell Physiol. 2021. PMID: 33570563 Review.
Cited by
-
Making and breaking of boron bridges in the pectic domain rhamnogalacturonan-II at apoplastic pH in vivo and in vitro.Plant J. 2023 Mar;113(6):1310-1329. doi: 10.1111/tpj.16112. Epub 2023 Feb 8. Plant J. 2023. PMID: 36658763 Free PMC article.
-
Structural and functional insights into the mechanism of action of plant borate transporters.Sci Rep. 2021 Jun 10;11(1):12328. doi: 10.1038/s41598-021-91763-6. Sci Rep. 2021. PMID: 34112901 Free PMC article.
-
Boron Toxicity and Deficiency in Agricultural Plants.Int J Mol Sci. 2020 Feb 20;21(4):1424. doi: 10.3390/ijms21041424. Int J Mol Sci. 2020. PMID: 32093172 Free PMC article. Review.
-
Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion.Ann Bot. 2022 Nov 17;130(5):703-715. doi: 10.1093/aob/mcac119. Ann Bot. 2022. PMID: 36112021 Free PMC article.
-
Storming the barricades of rhamnogalacturonan-II synthesis and function.Plant Cell. 2025 Jun 4;37(6):koaf088. doi: 10.1093/plcell/koaf088. Plant Cell. 2025. PMID: 40237333 Free PMC article. Review.
References
-
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. 2011. Plant cell walls: from chemistry to biology. New York, NY, USA: Garland Science.
-
- Blevins DG, Lukaszewski KM. 1998. Boron in plant structure and function. Annual Review of Plant Physiology and Plant Molecular Biology 49: 481–500. - PubMed
-
- Bolaños L, Lukaszewski K, Bonilla I, Blevins D. 2004. Why boron? Plant Physiology and Biochemistry 42: 907–912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

