Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity
- PMID: 26301736
- PMCID: PMC4676410
- DOI: 10.1021/acs.jmedchem.5b00709
Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity
Abstract
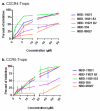
Earlier we reported the discovery and design of NBD-556 and their analogs which demonstrated their potential as HIV-1 entry inhibitors. However, progress in developing these inhibitors has been stymied by their CD4-agonist properties, an unfavorable trait for use as drug. Here, we demonstrate the successful conversion of a full CD4-agonist (NBD-556) through a partial CD4-agonist (NBD-09027), to a full CD4-antagonist (NBD-11021) by structure-based modification of the critical oxalamide midregion, previously thought to be intolerant of modification. NBD-11021 showed unprecedented neutralization breath for this class of inhibitors, with pan-neutralization against a panel of 56 Env-pseudotyped HIV-1 representing diverse subtypes of clinical isolates (IC50 as low as 270 nM). The cocrystal structure of NBD-11021 complexed to a monomeric HIV-1 gp120 core revealed its detail binding characteristics. The study is expected to provide a framework for further development of NBD series as HIV-1 entry inhibitors for clinical application against AIDS.
Figures


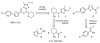

References
-
- Stevenson M. HIV-1 pathogenesis. Nat. Med. 2003;9:853–860. - PubMed
-
- Dalgleish AG, Beverly PCL, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. - PubMed
-
- Sattentau QJ, Weiss RA. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988;52:631–633. - PubMed
-
- Ray N, Doms RW. HIV-1 coreceptors and their inhibitors. Curr. Top. Microbiol. Immunol. 2006;303:97–120. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

