Adipocyte iron regulates leptin and food intake
- PMID: 26301810
- PMCID: PMC4588289
- DOI: 10.1172/JCI81860
Adipocyte iron regulates leptin and food intake
Abstract
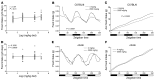
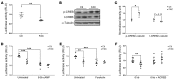
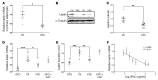
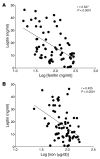
Dietary iron supplementation is associated with increased appetite. Here, we investigated the effect of iron on the hormone leptin, which regulates food intake and energy homeostasis. Serum ferritin was negatively associated with serum leptin in a cohort of patients with metabolic syndrome. Moreover, the same inverse correlation was observed in mice fed a high-iron diet. Adipocyte-specific loss of the iron exporter ferroportin resulted in iron loading and decreased leptin, while decreased levels of hepcidin in a murine hereditary hemochromatosis (HH) model increased adipocyte ferroportin expression, decreased adipocyte iron, and increased leptin. Treatment of 3T3-L1 adipocytes with iron decreased leptin mRNA in a dose-dependent manner. We found that iron negatively regulates leptin transcription via cAMP-responsive element binding protein activation (CREB activation) and identified 2 potential CREB-binding sites in the mouse leptin promoter region. Mutation of both sites completely blocked the effect of iron on promoter activity. ChIP analysis revealed that binding of phosphorylated CREB is enriched at these two sites in iron-treated 3T3-L1 adipocytes compared with untreated cells. Consistent with the changes in leptin, dietary iron content was also directly related to food intake, independently of weight. These findings indicate that levels of dietary iron play an important role in regulation of appetite and metabolism through CREB-dependent modulation of leptin expression.
Figures



Comment in
-
Hungry irony.J Clin Invest. 2015 Sep;125(9):3422-3. doi: 10.1172/JCI83193. Epub 2015 Aug 24. J Clin Invest. 2015. PMID: 26301806 Free PMC article.
References
-
- Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr. 1994;124(5):645–654. - PubMed
-
- Stoltzfus RJ, et al. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr. 2004;134(2):348–356. - PubMed
-
- Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. comparison of obese and nonobese subjects. Am J Clin Nutr. 1963;13:354–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

