Halting progressive neurodegeneration in advanced retinitis pigmentosa
- PMID: 26301813
- PMCID: PMC4588299
- DOI: 10.1172/JCI82462
Halting progressive neurodegeneration in advanced retinitis pigmentosa
Abstract
Hereditary retinal degenerative diseases, such as retinitis pigmentosa (RP), are characterized by the progressive loss of rod photoreceptors followed by loss of cones. While retinal gene therapy clinical trials demonstrated temporary improvement in visual function, this approach has yet to achieve sustained functional and anatomical rescue after disease onset in patients. The lack of sustained benefit could be due to insufficient transduction efficiency of viral vectors ("too little") and/or because the disease is too advanced ("too late") at the time therapy is initiated. Here, we tested the latter hypothesis and developed a mouse RP model that permits restoration of the mutant gene in all diseased photoreceptor cells, thereby ensuring sufficient transduction efficiency. We then treated mice at early, mid, or late disease stages. At all 3 time points, degeneration was halted and function was rescued for at least 1 year. Not only do our results demonstrate that gene therapy effectively preserves function after the onset of degeneration, our study also demonstrates that there is a broad therapeutic time window. Moreover, these results suggest that RP patients are treatable, despite most being diagnosed after substantial photoreceptor loss, and that gene therapy research must focus on improving transduction efficiency to maximize clinical impact.
Figures

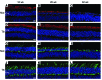


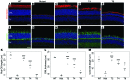
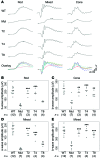
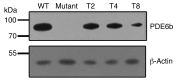
Comment in
-
It's never too late to save a photoreceptor.J Clin Invest. 2015 Sep;125(9):3424-6. doi: 10.1172/JCI83194. Epub 2015 Aug 24. J Clin Invest. 2015. PMID: 26301805 Free PMC article.
References
-
- Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34(5):1659–1676. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

