Dopaminergic and cholinergic learning mechanisms in nicotine addiction
- PMID: 26301866
- PMCID: PMC4564314
- DOI: 10.1111/nyas.12871
Dopaminergic and cholinergic learning mechanisms in nicotine addiction
Abstract
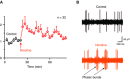
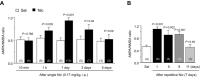
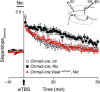
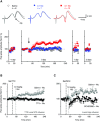
Nicotine addiction drives tobacco use by one billion people worldwide, causing nearly six million deaths a year. Nicotine binds to nicotinic acetylcholine receptors that are normally activated by the endogenous neurotransmitter acetylcholine. The widespread expression of nicotinic receptors throughout the nervous system accounts for the diverse physiological effects triggered by nicotine. A crucial influence of nicotine is on the synaptic mechanisms underlying learning that contribute to the addiction process. Here, we focus on the acquisition phase of smoking addiction and review animal model studies on how nicotine modifies dopaminergic and cholinergic signaling in key nodes of the reinforcement circuitry: ventral tegmental area, nucleus accumbens (NAc), amygdala, and hippocampus. Capitalizing on mechanisms that subserve natural rewards, nicotine activates midbrain dopamine neurons directly and indirectly, and nicotine causes dopamine release in very broad target areas throughout the brain, including the NAc, amygdala, and hippocampus. In addition, nicotine orchestrates local changes within those target structures, alters the release of virtually all major neurotransmitters, and primes the nervous system to the influence of other addictive drugs. Hence, understanding how nicotine affects the circuitry for synaptic plasticity and learning may aid in developing reasoned therapies to treat nicotine addiction.
Keywords: VTA; acetylcholine; dopamine; hippocampus; memory; nucleus accumbens.
© 2015 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals Inc. on behalf of The New York Academy of Sciences.
Figures




Similar articles
-
Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction.Learn Mem. 2004 Jan-Feb;11(1):60-9. doi: 10.1101/lm.70004. Learn Mem. 2004. PMID: 14747518 Free PMC article.
-
α6-Containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity.Neuroscience. 2015 Sep 24;304:161-75. doi: 10.1016/j.neuroscience.2015.07.052. Epub 2015 Jul 23. Neuroscience. 2015. PMID: 26210579 Free PMC article.
-
Nicotine inhibits the VTA-to-amygdala dopamine pathway to promote anxiety.Neuron. 2021 Aug 18;109(16):2604-2615.e9. doi: 10.1016/j.neuron.2021.06.013. Epub 2021 Jul 8. Neuron. 2021. PMID: 34242565
-
Synaptic plasticity and nicotine addiction.Neuron. 2001 Aug 16;31(3):349-52. doi: 10.1016/s0896-6273(01)00379-8. Neuron. 2001. PMID: 11516393 Review.
-
Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction.Eur J Pharmacol. 2003 Nov 7;480(1-3):117-23. doi: 10.1016/j.ejphar.2003.08.099. Eur J Pharmacol. 2003. PMID: 14623355 Review.
Cited by
-
Trace amine-associated receptor 1 and drug abuse.Adv Pharmacol. 2022;93:373-401. doi: 10.1016/bs.apha.2021.10.005. Epub 2021 Nov 11. Adv Pharmacol. 2022. PMID: 35341572 Free PMC article.
-
Systemic nicotine enhances opioid self-administration and modulates the formation of opioid-associated memories partly through actions within the insular cortex.Sci Rep. 2021 Feb 8;11(1):3321. doi: 10.1038/s41598-021-81955-5. Sci Rep. 2021. PMID: 33558613 Free PMC article.
-
The Association Between Chronic Tobacco Smoking and Brain Alterations in Schizophrenia: A Systematic Review of Magnetic Resonance Imaging Studies.Schizophr Bull. 2025 May 8;51(3):608-624. doi: 10.1093/schbul/sbae088. Schizophr Bull. 2025. PMID: 38824451 Free PMC article.
-
Neurobiological and Neurophysiological Mechanisms Underlying Nicotine Seeking and Smoking Relapse.Mol Neuropsychiatry. 2019 Feb;4(4):169-189. doi: 10.1159/000494799. Epub 2018 Nov 19. Mol Neuropsychiatry. 2019. PMID: 30815453 Free PMC article. Review.
-
Role of trace amine-associated receptor 1 in nicotine's behavioral and neurochemical effects.Neuropsychopharmacology. 2018 Nov;43(12):2435-2444. doi: 10.1038/s41386-018-0017-9. Epub 2018 Feb 5. Neuropsychopharmacology. 2018. PMID: 29472642 Free PMC article.
References
-
- Ng, M. et al 2014. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311: 183–192. - PubMed
-
- Dani, J.A. , Kosten T.R. & Benowitz N.L.. 2009. “The pharmacology of nicotine and tobacco.” In Principles of Addiction Medicine, 4th ed. Ries R.K., Fiellin D.A., Miller S.C. & Saitz R., Eds.: 179–192. Wolters Kluwer, Lippincott, Williams & Wilkins.
-
- World Health Organization. 2015. Tobacco fact sheet 339. July 6, 2015. Accessed Aug. 5, 2015., authors http://www.who.int/mediacentre/factsheets/fs339/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

