Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications
- PMID: 26302388
- PMCID: PMC4557396
- DOI: 10.1148/radiol.2015142631
Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications
Abstract
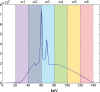
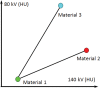
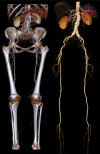
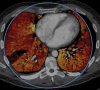
In x-ray computed tomography (CT), materials having different elemental compositions can be represented by identical pixel values on a CT image (ie, CT numbers), depending on the mass density of the material. Thus, the differentiation and classification of different tissue types and contrast agents can be extremely challenging. In dual-energy CT, an additional attenuation measurement is obtained with a second x-ray spectrum (ie, a second "energy"), allowing the differentiation of multiple materials. Alternatively, this allows quantification of the mass density of two or three materials in a mixture with known elemental composition. Recent advances in the use of energy-resolving, photon-counting detectors for CT imaging suggest the ability to acquire data in multiple energy bins, which is expected to further improve the signal-to-noise ratio for material-specific imaging. In this review, the underlying motivation and physical principles of dual- or multi-energy CT are reviewed and each of the current technical approaches is described. In addition, current and evolving clinical applications are introduced.
Figures




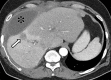
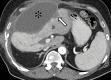
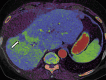
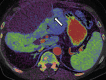
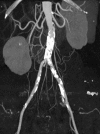
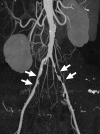


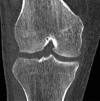



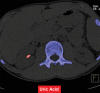
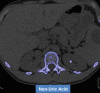
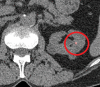
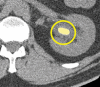

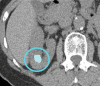


References
-
- Hounsfield GN. Computerized transverse axial scanning (tomography). I. Description of system. Br J Radiol 1973;46(552):1016–1022. - PubMed
-
- Alvarez RE, Macovski A. Energy-selective reconstructions in x-ray computerized tomography. Phys Med Biol 1976;21(5):733–744. - PubMed
-
- Macovski A, Alvarez RE, Chan JL, Stonestrom JP, Zatz LM. Energy dependent reconstruction in x-ray computerized tomography. Comput Biol Med 1976;6(4):325–336. - PubMed
-
- Kalender WA, Klotz E, Suess C. Vertebral bone mineral analysis: an integrated approach with CT. Radiology 1987;164(2):419–423. - PubMed
-
- Kalender WA, Perman WH, Vetter JR, Klotz E. Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies. Med Phys 1986;13(3):334–339. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

