Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease
- PMID: 26302439
- PMCID: PMC4944824
- DOI: 10.1001/jamaneurol.2015.1807
Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease
Abstract
Importance: Alzheimer disease (AD) is the most common neurodegenerative disorder and lacks effective disease-modifying therapies. In 2001, we initiated a clinical trial of nerve growth factor (NGF) gene therapy in AD, the first effort at gene delivery in an adult neurodegenerative disorder. This program aimed to determine whether a nervous system growth factor prevents or reduces cholinergic neuronal degeneration in patients with AD. We present postmortem findings in 10 patients with survival times ranging from 1 to 10 years after treatment.
Objective: To determine whether degenerating neurons in AD retain an ability to respond to a nervous system growth factor delivered after disease onset.
Design, setting, and participants: Patients in this anatomicopathological study were enrolled in clinical trials from March 2001 to October 2012 at the University of California, San Diego, Medical Center in La Jolla. Ten patients with early AD underwent NGF gene therapy using ex vivo or in vivo gene transfer. The brains of all 8 patients in the first phase 1 ex vivo trial and of 2 patients in a subsequent phase 1 in vivo trial were examined.
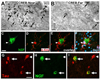
Main outcomes and measures: Brains were immunolabeled to evaluate in vivo gene expression, cholinergic neuronal responses to NGF, and activation of NGF-related cell signaling. In 2 patients, NGF protein levels were measured by enzyme-linked immunosorbent assay.
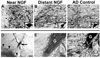
Results: Among 10 patients, degenerating neurons in the AD brain responded to NGF. All patients exhibited a trophic response to NGF in the form of axonal sprouting toward the NGF source. Comparing treated and nontreated sides of the brain in 3 patients who underwent unilateral gene transfer, cholinergic neuronal hypertrophy occurred on the NGF-treated side (P < .05). Activation of cellular signaling and functional markers was present in 2 patients who underwent adeno-associated viral vectors (serotype 2)-mediated NGF gene transfer. Neurons exhibiting tau pathology and neurons free of tau expressed NGF, indicating that degenerating cells can be infected with therapeutic genes, with resultant activation of cell signaling. No adverse pathological effects related to NGF were observed.
Conclusions and relevance: These findings indicate that neurons of the degenerating brain retain the ability to respond to growth factors with axonal sprouting, cell hypertrophy, and activation of functional markers. Sprouting induced by NGF persists for 10 years after gene transfer. Growth factor therapy appears safe over extended periods and merits continued testing as a means of treating neurodegenerative disorders.
Figures


Comment in
-
Alzheimer disease: NGF gene therapy activates neurons in the AD patient brain.Nat Rev Neurol. 2015 Oct;11(10):548. doi: 10.1038/nrneurol.2015.170. Epub 2015 Sep 8. Nat Rev Neurol. 2015. PMID: 26347367 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

