Spatio-temporal Genetic Structuring of Leishmania major in Tunisia by Microsatellite Analysis
- PMID: 26302440
- PMCID: PMC4547700
- DOI: 10.1371/journal.pntd.0004017
Spatio-temporal Genetic Structuring of Leishmania major in Tunisia by Microsatellite Analysis
Abstract
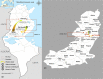
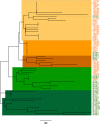
In Tunisia, cases of zoonotic cutaneous leishmaniasis caused by Leishmania major are increasing and spreading from the south-west to new areas in the center. To improve the current knowledge on L. major evolution and population dynamics, we performed multi-locus microsatellite typing of human isolates from Tunisian governorates where the disease is endemic (Gafsa, Kairouan and Sidi Bouzid governorates) and collected during two periods: 1991-1992 and 2008-2012. Analysis (F-statistics and Bayesian model-based approach) of the genotyping results of isolates collected in Sidi Bouzid in 1991-1992 and 2008-2012 shows that, over two decades, in the same area, Leishmania parasites evolved by generating genetically differentiated populations. The genetic patterns of 2008-2012 isolates from the three governorates indicate that L. major populations did not spread gradually from the south to the center of Tunisia, according to a geographical gradient, suggesting that human activities might be the source of the disease expansion. The genotype analysis also suggests previous (Bayesian model-based approach) and current (F-statistics) flows of genotypes between governorates and districts. Human activities as well as reservoir dynamics and the effects of environmental changes could explain how the disease progresses. This study provides new insights into the evolution and spread of L. major in Tunisia that might improve our understanding of the parasite flow between geographically and temporally distinct populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Déperet C, Boinet E (1884) Du bouton de Gafsa au champ de Sathomay. Arch Méd Pharm Mil 3: 296–302.
-
- Ashford R (2000) The leishmaniases as emerging and reemerging zoonoses. International Journal for Parasitology 30: 1269–1281. - PubMed
-
- Ben Salah A, Zakraoui H, Zaatour A, Ftaiti A, Zaafouri B, et al. (1995) A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am J Trop Med Hyg 53: 162–166. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

