Stress exposure, food intake and emotional state
- PMID: 26303312
- PMCID: PMC4843770
- DOI: 10.3109/10253890.2015.1062981
Stress exposure, food intake and emotional state
Abstract
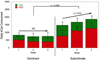
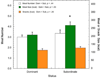
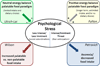
This manuscript summarizes the proceedings of the symposium entitled, "Stress, Palatable Food and Reward", that was chaired by Drs. Linda Rinaman and Yvonne Ulrich-Lai at the 2014 Neurobiology of Stress Workshop held in Cincinnati, OH. This symposium comprised research presentations by four neuroscientists whose work focuses on the biological bases for complex interactions among stress, food intake and emotion. First, Dr Ulrich-Lai describes her rodent research exploring mechanisms by which the rewarding properties of sweet palatable foods confer stress relief. Second, Dr Stephanie Fulton discusses her work in which excessive, long-term intake of dietary lipids, as well as their subsequent withdrawal, promotes stress-related outcomes in mice. Third, Dr Mark Wilson describes his group's research examining the effects of social hierarchy-related stress on food intake and diet choice in group-housed female rhesus macaques, and compared the data from monkeys to results obtained in analogous work using rodents. Finally, Dr Gorica Petrovich discusses her research program that is aimed at defining cortical-amygdalar-hypothalamic circuitry responsible for curbing food intake during emotional threat (i.e. fear anticipation) in rats. Their collective results reveal the complexity of physiological and behavioral interactions that link stress, food intake and emotional state, and suggest new avenues of research to probe the impact of genetic, metabolic, social, experiential and environmental factors on these interactions.
Keywords: Anorexia; HPA axis; anxiety; comfort food; depression; obesity.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


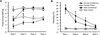
Similar articles
-
Diet matters: Glucocorticoid-related neuroadaptations associated with calorie intake in female rhesus monkeys.Psychoneuroendocrinology. 2018 May;91:169-178. doi: 10.1016/j.psyneuen.2018.03.008. Epub 2018 Mar 14. Psychoneuroendocrinology. 2018. PMID: 29567621 Free PMC article.
-
Role of addiction and stress neurobiology on food intake and obesity.Biol Psychol. 2018 Jan;131:5-13. doi: 10.1016/j.biopsycho.2017.05.001. Epub 2017 May 4. Biol Psychol. 2018. PMID: 28479142 Free PMC article. Review.
-
HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption.Physiol Behav. 2011 Apr 18;103(1):104-10. doi: 10.1016/j.physbeh.2010.12.011. Epub 2010 Dec 17. Physiol Behav. 2011. PMID: 21168428 Free PMC article.
-
Limited cheese intake reduces HPA axis and behavioral stress responses in male rats.Physiol Behav. 2021 Dec 1;242:113614. doi: 10.1016/j.physbeh.2021.113614. Epub 2021 Oct 1. Physiol Behav. 2021. PMID: 34600921 Free PMC article.
-
Stress and eating behaviors.Minerva Endocrinol. 2013 Sep;38(3):255-67. Minerva Endocrinol. 2013. PMID: 24126546 Free PMC article. Review.
Cited by
-
Secretin Regulates Excitatory GABAergic Neurotransmission to GnRH Neurons via Retrograde NO Signaling Pathway in Mice.Front Cell Neurosci. 2019 Aug 23;13:371. doi: 10.3389/fncel.2019.00371. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31507377 Free PMC article.
-
Parabrachial Interleukin-6 Reduces Body Weight and Food Intake and Increases Thermogenesis to Regulate Energy Metabolism.Cell Rep. 2019 Mar 12;26(11):3011-3026.e5. doi: 10.1016/j.celrep.2019.02.044. Cell Rep. 2019. PMID: 30865890 Free PMC article.
-
Association between Perceived Psychological Stress and Exercise Behaviors: A Cross-Sectional Study Using the Survey of National Physical Fitness.Life (Basel). 2023 Oct 14;13(10):2059. doi: 10.3390/life13102059. Life (Basel). 2023. PMID: 37895440 Free PMC article.
-
A cross-species assay demonstrates that reward responsiveness is enduringly impacted by adverse, unpredictable early-life experiences.Neuropsychopharmacology. 2022 Feb;47(3):767-775. doi: 10.1038/s41386-021-01250-9. Epub 2021 Dec 17. Neuropsychopharmacology. 2022. PMID: 34921225 Free PMC article.
-
Locus coeruleus anchors a trisynaptic circuit controlling fear-induced suppression of feeding.Neuron. 2021 Mar 3;109(5):823-838.e6. doi: 10.1016/j.neuron.2020.12.023. Epub 2021 Jan 20. Neuron. 2021. PMID: 33476548 Free PMC article.
References
-
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. - PubMed
-
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 2013.
-
- Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS, Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40:1761–1768. - PubMed
-
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK078906/DK/NIDDK NIH HHS/United States
- DK100685/DK/NIDDK NIH HHS/United States
- MH059911/MH/NIMH NIH HHS/United States
- R01 DK096983/DK/NIDDK NIH HHS/United States
- ODP51011132/PHS HHS/United States
- R01 MH059911/MH/NIMH NIH HHS/United States
- MH081816/MH/NIMH NIH HHS/United States
- R01 DK085721/DK/NIDDK NIH HHS/United States
- R03 DK089018/DK/NIDDK NIH HHS/United States
- R01 MH079100/MH/NIMH NIH HHS/United States
- R01 DK091425/DK/NIDDK NIH HHS/United States
- MH79100/MH/NIMH NIH HHS/United States
- DK096983/DK/NIDDK NIH HHS/United States
- R01 MH081816/MH/NIMH NIH HHS/United States
- R01 DK100685/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
