Phobalysin, a Small β-Pore-Forming Toxin of Photobacterium damselae subsp. damselae
- PMID: 26303391
- PMCID: PMC4598394
- DOI: 10.1128/IAI.00277-15
Phobalysin, a Small β-Pore-Forming Toxin of Photobacterium damselae subsp. damselae
Abstract
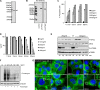
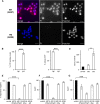
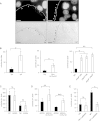
Photobacterium damselae subsp. damselae, an important pathogen of marine animals, may also cause septicemia or hyperaggressive necrotizing fasciitis in humans. We previously showed that hemolysin genes are critical for virulence of this organism in mice and fish. In the present study, we characterized the hlyA gene product, a putative small β-pore-forming toxin, and termed it phobalysin P (PhlyP), for "photobacterial lysin encoded on a plasmid." PhlyP formed stable oligomers and small membrane pores, causing efflux of K(+), with no significant leakage of lactate dehydrogenase but entry of vital dyes. The latter feature distinguished PhlyP from the related Vibrio cholerae cytolysin. Attack by PhlyP provoked a loss of cellular ATP, attenuated translation, and caused profound morphological changes in epithelial cells. In coculture experiments with epithelial cells, Photobacterium damselae subsp. damselae led to rapid hemolysin-dependent membrane permeabilization. Unexpectedly, hemolysins also promoted the association of P. damselae subsp. damselae with epithelial cells. The collective observations of this study suggest that membrane-damaging toxins commonly enhance bacterial adherence.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

