Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer
- PMID: 26303618
- PMCID: PMC4548900
- DOI: 10.1186/s12967-015-0632-8
Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer
Abstract
Background: NK cells can destroy tumor cells without prior sensitization or immunization. Tumors often lose expression of MHC molecules and/or antigens. However, NK cells can lyse tumor cells in a non-MHC-restricted manner and independent of the expression of tumor-associated antigens. NK cells are therefore considered ideal for adoptive cancer immunotherapy; however the difficulty of obtaining large numbers of fully functional NK cells that are safe to administer deters its clinical use. This phase I clinical trial seeks to address this obstacle by first developing a novel system that expands large numbers of highly activated clinical grade NK cells, and second, determining if these cells are safe in a mono-treatment so they can be combined with other reagents in the next round of clinical trials.
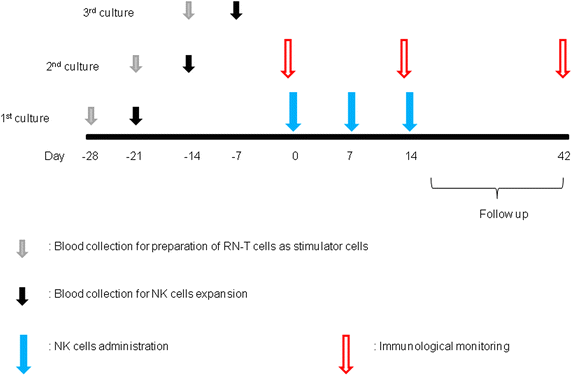
Methods: Patients with unresectable, locally advanced and/or metastatic digestive cancer who did not succeed with standard therapy were enrolled. NK cells were expanded ex vivo by stimulating PBMCs with OK432, IL-2, and modified FN-CH296 induced T cells. Patients were administered autologous natural killer cell three times weekly via intravenous infusions in a dose-escalating manner (dose 0.5 × 10(9), 1.0 × 10(9), 2.0 × 10(9) cells/injection, three patients/one cohort).
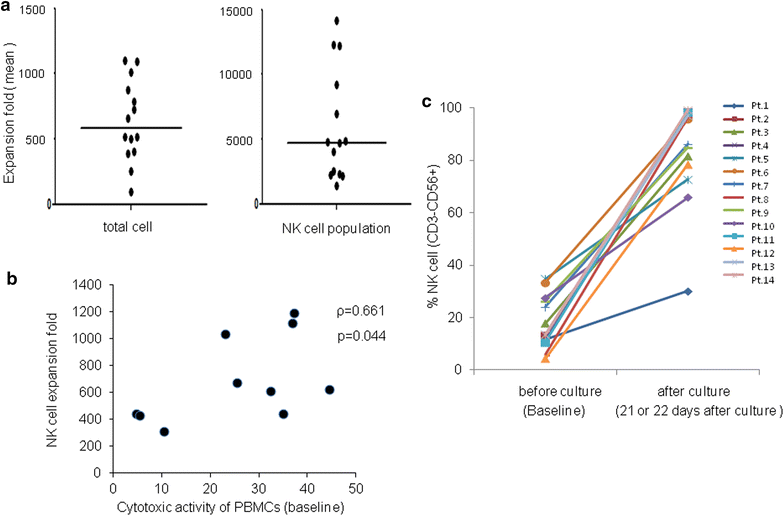
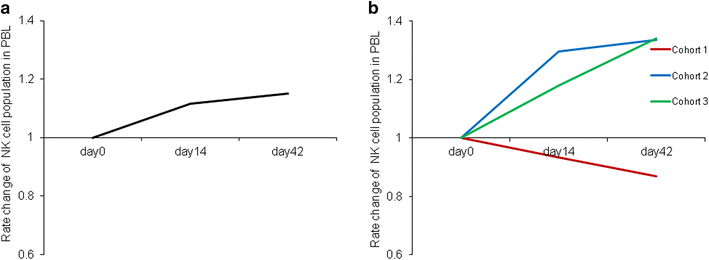
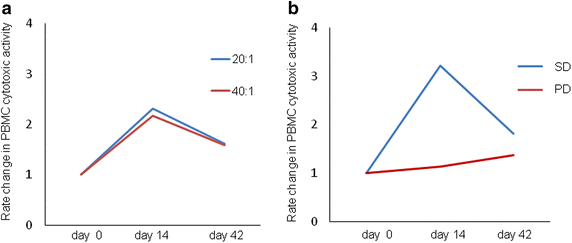
Results: Total cell population had a median expansion of 586-fold (range 95-1102), with a significantly pure (90.96 %) NK cell population. Consequently, NK cells were expanded to approximately 4720-fold (range 1372-14,116) with cells being highly lytic in vitro and strongly expressing functional markers such as NKG2D and CD16. This NK cell therapy was very well tolerated with no severe adverse events. Although no clinical responses were observed, cytotoxicity of peripheral blood was elevated approximately twofolds up to 4 weeks post the last transfer.
Conclusion: We successfully generated large numbers of activated NK cells from small quantities of blood without prior purification of the cells. We also determined that the expanded cells were safe to administer in a monotherapy and are suitable for the next round of clinical trials where their efficacy will be tested combined with other reagents.
Trial registration: UMIN UMIN000007527.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

