Towards a systems view of IBS
- PMID: 26303675
- PMCID: PMC5001844
- DOI: 10.1038/nrgastro.2015.121
Towards a systems view of IBS
Abstract
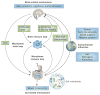
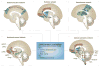
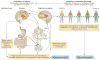
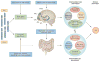
Despite an extensive body of reported information about peripheral and central mechanisms involved in the pathophysiology of IBS symptoms, no comprehensive disease model has emerged that would guide the development of novel, effective therapies. In this Review, we will first describe novel insights into some key components of brain-gut interactions, starting with the emerging findings of distinct functional and structural brain signatures of IBS. We will then point out emerging correlations between these brain networks and genomic, gastrointestinal, immune and gut-microbiome-related parameters. We will incorporate this new information, as well as the reported extensive literature on various peripheral mechanisms, into a systems-based disease model of IBS, and discuss the implications of such a model for improved understanding of the disorder, and for the development of more-effective treatment approaches in the future.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Longstreth GF, et al. In: ROME III: The Functional Gastrointestinal Disorders. Drossman DA, et al., editors. Degnon Associates; 2006. pp. 487–556.
-
- Mayer EA, Bushnell MC. In: Functional Pain Syndromes: Presentation and Pathophysiology. Mayer EA, Bushnell MC, editors. IASP Press; 2009. pp. 531–565.
-
- Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. - PubMed
-
- Hughes PA, et al. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. - PubMed
-
- Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

