Mindfulness-based cognitive therapy for inflammatory bowel disease patients: findings from an exploratory pilot randomised controlled trial
- PMID: 26303912
- PMCID: PMC4549082
- DOI: 10.1186/s13063-015-0909-5
Mindfulness-based cognitive therapy for inflammatory bowel disease patients: findings from an exploratory pilot randomised controlled trial
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic gastrointestinal condition with a relapsing disease course. Managing the relapsing nature of the disease causes daily stress for IBD patients; thus, IBD patients report higher rates of depression and anxiety than the general population. Mindfulness-based Cognitive Therapy (MBCT) is an evidence-based psychological program designed to help manage depressive and stress symptoms. There has been no randomized controlled trial (RCT) testing the use of MBCT in IBD patients. The purpose of this pilot study is to test the trial methodology and assess the feasibility of conducting a large RCT testing the effectiveness of MBCT in IBD.
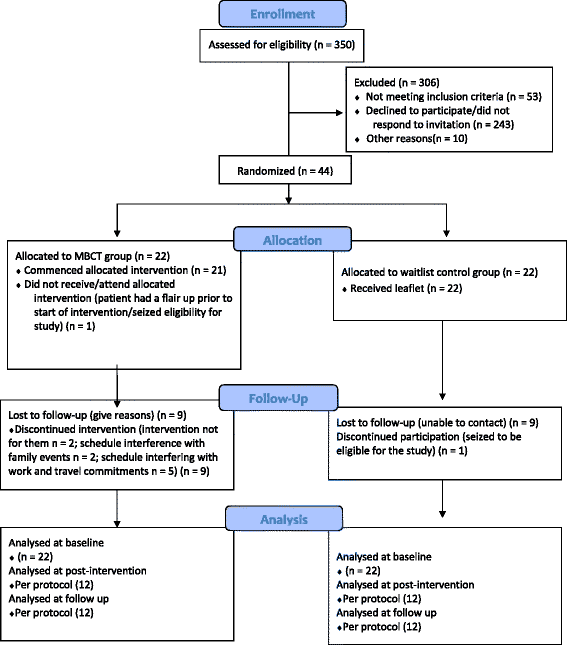
Methods: The IBD patients, who were recruited from gastroenterology outpatient clinics at two Scottish NHS Boards, were randomly allocated to an MBCT intervention group (n = 22) or a wait-list control group (n = 22). The MBCT intervention consisted of 16 hours of structured group training over 8 consecutive weeks plus guided home practice and follow-up sessions. The wait-list group received a leaflet entitled 'Staying well with IBD'. All participants completed a baseline, post-intervention and 6-month follow up assessment. The key objectives were to assess patient eligibility and recruitment/dropout rate, to calculate initial estimates of parameters to the proposed outcome measures (depression, anxiety, disease activity, dispositional mindfulness and quality of life) and to estimate sample size for a future large RCT.
Results: In total, 350 patients were assessed for eligibility. Of these, 44 eligible patients consented to participate. The recruitment rate was 15%, with main reasons for ineligibility indicated as follows: non-response to invitation, active disease symptoms, planned surgery or incompatibility with group schedule. There was a higher than expected dropout rate of 44%. Initial estimates of parameters to the proposed outcomes at post-intervention and follow-up showed a significant improvement of scores in the MBCT group when compared to the control for depression, trait anxiety and dispositional mindfulness. The sample-size calculation was guided by estimates of clinically important effects in depression scores.
Conclusions: This pilot study suggests that a multicentre randomized clinical trial testing the effectiveness of MBCT for IBD patients is feasible with some changes to the protocol. Improvement in depression, trait anxiety and dispositional mindfulness scores are promising when coupled with patients reporting a perceived improvement of their quality of life.
Trial registration: ISRCTN27934462. 2 August 2013.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical