N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway
- PMID: 26303969
- PMCID: PMC4911206
- DOI: 10.5483/bmbrep.2015.48.11.112
N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway
Abstract
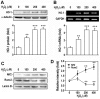
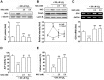
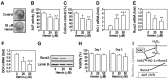
There are controversial findings regarding the roles of nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway on bone metabolism under oxidative stress. We investigated how Nrf2/HO-1 pathway affects osteoblast differentiation of MC3T3-E1 cells in response to hydrogen peroxide (H2O2), N-acetyl cysteine (NAC), or both. Exposing the cells to H2O2 decreased the alkaline phosphatase activity, calcium accumulation, and expression of osteoblast markers, such as osteocalcin and runt-related transcription factor-2. In contrast, H2O2 treatment increased the expression of Nrf2 and HO-1 in the cells. Treatment with hemin, a chemical HO-1 inducer, mimicked the inhibitory effect of H2O2 on osteoblast differentiation by increasing the HO-1 expression and decreasing the osteogenic marker genes. Pretreatment with NAC restored all changes induced by H2O2 to near normal levels in the cells. Collectively, our findings suggest that H2O2-mediated activation of Nrf2/HO-1 pathway negatively regulates the osteoblast differentiation, which is inhibited by NAC.
Figures

Similar articles
-
The Role of NRF2 in Bone Metabolism - Friend or Foe?Front Endocrinol (Lausanne). 2022 Feb 23;13:813057. doi: 10.3389/fendo.2022.813057. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35282459 Free PMC article. Review.
-
Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway.Oncotarget. 2017 Feb 28;8(9):14680-14692. doi: 10.18632/oncotarget.14747. Oncotarget. 2017. PMID: 28122344 Free PMC article.
-
Irradiation inhibits the maturation and mineralization of osteoblasts via the activation of Nrf2/HO-1 pathway.Mol Cell Biochem. 2015 Dec;410(1-2):255-66. doi: 10.1007/s11010-015-2559-z. Epub 2015 Sep 7. Mol Cell Biochem. 2015. PMID: 26346162
-
Cytoprotective effect of Fufang Lurong Jiangu capsule against hydrogen peroxide-induced oxidative stress in bone marrow stromal cell-derived osteoblasts through the Nrf2/HO-1 signaling pathway.Biomed Pharmacother. 2020 Jan;121:109676. doi: 10.1016/j.biopha.2019.109676. Epub 2019 Nov 25. Biomed Pharmacother. 2020. PMID: 31810119
-
Glucose oxidase facilitates osteogenic differentiation and mineralization of embryonic stem cells through the activation of Nrf2 and ERK signal transduction pathways.Mol Cell Biochem. 2016 Aug;419(1-2):157-63. doi: 10.1007/s11010-016-2760-8. Epub 2016 Jul 19. Mol Cell Biochem. 2016. PMID: 27431005
Cited by
-
Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice.Nutrients. 2025 Jun 26;17(13):2116. doi: 10.3390/nu17132116. Nutrients. 2025. PMID: 40647221 Free PMC article.
-
The Role of NRF2 in Bone Metabolism - Friend or Foe?Front Endocrinol (Lausanne). 2022 Feb 23;13:813057. doi: 10.3389/fendo.2022.813057. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35282459 Free PMC article. Review.
-
Protective effect of 3-(naphthalen-2-yl(propoxy)methyl)azetidine hydrochloride on hypoxia-induced toxicity by suppressing microglial activation in BV-2 cells.BMB Rep. 2016 Dec;49(12):687-692. doi: 10.5483/bmbrep.2016.49.12.169. BMB Rep. 2016. PMID: 27756444 Free PMC article.
-
Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway.Oncotarget. 2017 Feb 28;8(9):14680-14692. doi: 10.18632/oncotarget.14747. Oncotarget. 2017. PMID: 28122344 Free PMC article.
-
Low concentrations of clarithromycin upregulate cellular antioxidant enzymes and phosphorylation of extracellular signal-regulated kinase in human small airway epithelial cells.J Pharm Health Care Sci. 2018 Sep 3;4:23. doi: 10.1186/s40780-018-0120-4. eCollection 2018. J Pharm Health Care Sci. 2018. PMID: 30186615 Free PMC article.
References
-
- Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. (2008);46:1550–1555. - PubMed

