UCP-3 uncoupling protein confers hypoxia resistance to renal epithelial cells and is upregulated in renal cell carcinoma
- PMID: 26304588
- PMCID: PMC4548255
- DOI: 10.1038/srep13450
UCP-3 uncoupling protein confers hypoxia resistance to renal epithelial cells and is upregulated in renal cell carcinoma
Abstract
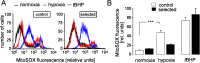
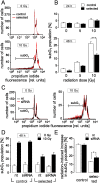
Tumor cells can adapt to a hostile environment with reduced oxygen supply. The present study aimed to identify mechanisms that confer hypoxia resistance. Partially hypoxia/reoxygenation (H/R)-resistant proximal tubular (PT) cells were selected by exposing PT cultures to repetitive cycles of H/R. Thereafter, H/R-induced changes in mRNA and protein expression, inner mitochondrial membrane potential (ΔΨ(m)), formation of superoxide, and cell death were compared between H/R-adapted and control PT cultures. As a result, H/R-adapted PT cells exhibited lower H/R-induced hyperpolarization of ΔΨ(m) and produced less superoxide than the control cultures. Consequently, H/R triggered ΔΨ(m) break-down and DNA degradation in a lower percentage of H/R-adapted than control PT cells. Moreover, H/R induced upregulation of mitochondrial uncoupling protein-3 (UCP-3) in H/R-adapted PT but not in control cultures. In addition, ionizing radiation killed a lower percentage of H/R-adapted as compared to control cells suggestive of an H/R-radiation cross-resistance developed by the selection procedure. Knockdown of UCP-3 decreased H/R- and radioresitance of the H/R-adapted cells. Finally, UCP-3 protein abundance of PT-derived clear cell renal cell carcinoma and normal renal tissue was compared in human specimens indicating upregulation of UCP-3 during tumor development. Combined, our data suggest functional significance of UCP-3 for H/R resistance.
Figures





References
-
- Huber S. M., Misovic M., Mayer C., Rodemann H. P. & Dittmann K. EGFR-mediated stimulation of sodium/glucose cotransport promotes survival of irradiated human A549 lung adenocarcinoma cells. Radiother Oncol 103, 373–379 (2012). - PubMed
-
- Dittmann K., Mayer C., Rodemann H. P. & Huber S. M. EGFR cooperates with glucose transporter SGLT1 to enable chromatin remodeling in response to ionizing radiation. Radiother Oncol 107, 247–251 (2013). - PubMed
-
- Huber S. M. Oncochannels. Cell Calcium 53, 241–255 (2013). - PubMed
-
- Duranton C. et al. CFTR is involved in the fine tuning of intracellular redox status: physiological implications in cystic fibrosis. Am J Pathol 181, 1367–1377 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

