Glyceraldehyde caused Alzheimer's disease-like alterations in diagnostic marker levels in SH-SY5Y human neuroblastoma cells
- PMID: 26304819
- PMCID: PMC4548441
- DOI: 10.1038/srep13313
Glyceraldehyde caused Alzheimer's disease-like alterations in diagnostic marker levels in SH-SY5Y human neuroblastoma cells
Abstract
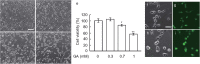
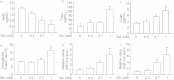
Clinical evidence has implicated diabetes mellitus as one of the risk factors for the development and progression of Alzheimer's disease (AD). However, the neurotoxic pathway activated due to abnormalities in glucose metabolism has not yet been identified in AD. In order to investigate the relationship between impaired cerebral glucose metabolism and the pathophysiology of AD, SH-SY5Y human neuroblastoma cells were exposed to glyceraldehyde (GA), an inhibitor of glycolysis. GA induced the production of GA-derived advanced glycation end-products (GA-AGEs) and cell apoptosis, glycolytic inhibition, decreases in the medium concentrations of diagnostic markers of AD, such as amyloid β 1-42 (Aβ42), and increases in tau phosphorylation. These results suggest that the production of GA-AGEs and/or inhibition of glycolysis induce AD-like alterations, and this model may be useful for examining the pathophysiology of AD.
Figures

References
-
- Takeuchi M. et al.. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer Res. 1, 39–46 (2004). - PubMed
-
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci. 17, 489–517 (1994). - PubMed
-
- Takeuchi M. & Makita Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr. Mol. Med. 1, 305–315 (2001). - PubMed
-
- Takeuchi M. & Yamagishi S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 63, 449–452 (2004). - PubMed
-
- Takeuchi M., Takino J. & Yamagishi S. Involvement of the toxic AGEs (TAGE)-RAGE system in the pathogenesis of diabetic vascular complications: A novel therapeutic strategy. Curr. Drug Targets 11, 1468–1482 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

