Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells
- PMID: 26304831
- PMCID: PMC4548446
- DOI: 10.1038/srep13317
Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells
Abstract
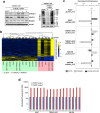
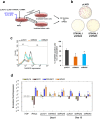
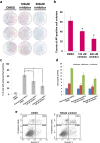
Many studies have suggested the significance of glycosyltransferase-mediated macromolecule glycosylation in the regulation of pluripotent states in human pluripotent stem cells (hPSCs). Here, we observed that the sialyltransferase ST6GAL1 was preferentially expressed in undifferentiated hPSCs compared to non-pluripotent cells. A lectin which preferentially recognizes α-2,6 sialylated galactosides showed strong binding reactivity with undifferentiated hPSCs and their glycoproteins, and did so to a much lesser extent with differentiated cells. In addition, downregulation of ST6GAL1 in undifferentiated hPSCs led to a decrease in POU5F1 (also known as OCT4) protein and significantly altered the expression of many genes that orchestrate cell morphogenesis during differentiation. The induction of cellular pluripotency in somatic cells was substantially impeded by the shRNA-mediated suppression of ST6GAL1, partially through interference with the expression of endogenous POU5F1 and SOX2. Targeting ST6GAL1 activity with a sialyltransferase inhibitor during cell reprogramming resulted in a dose-dependent reduction in the generation of human induced pluripotent stem cells (hiPSCs). Collectively, our data indicate that ST6GAL1 plays an important role in the regulation of pluripotency and differentiation in hPSCs, and the pluripotent state in human cells can be modulated using pharmacological tools to target sialyltransferase activity.
Figures

Similar articles
-
Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis.Cell Res. 2011 Nov;21(11):1551-63. doi: 10.1038/cr.2011.148. Epub 2011 Sep 6. Cell Res. 2011. PMID: 21894191 Free PMC article.
-
Reptin regulates pluripotency of embryonic stem cells and somatic cell reprogramming through Oct4-dependent mechanism.Stem Cells. 2014 Dec;32(12):3126-36. doi: 10.1002/stem.1827. Stem Cells. 2014. PMID: 25185564
-
ST6GAL1 negatively regulates monocyte transendothelial migration and atherosclerosis development.Biochem Biophys Res Commun. 2018 Jun 2;500(2):249-255. doi: 10.1016/j.bbrc.2018.04.053. Epub 2018 Apr 16. Biochem Biophys Res Commun. 2018. PMID: 29654763
-
Regulation of ST6GAL1 sialyltransferase expression in cancer cells.Glycobiology. 2021 Jun 3;31(5):530-539. doi: 10.1093/glycob/cwaa110. Glycobiology. 2021. PMID: 33320246 Free PMC article. Review.
-
The 'sweet' spot of cellular pluripotency: protein glycosylation in human pluripotent stem cells and its applications in regenerative medicine.Expert Opin Biol Ther. 2015 May;15(5):679-87. doi: 10.1517/14712598.2015.1021329. Epub 2015 Mar 3. Expert Opin Biol Ther. 2015. PMID: 25736263 Review.
Cited by
-
ST8SIA4-Dependent Polysialylation is Part of a Developmental Program Required for Germ Layer Formation from Human Pluripotent Stem Cells.Stem Cells. 2016 Jul;34(7):1742-52. doi: 10.1002/stem.2379. Epub 2016 May 3. Stem Cells. 2016. PMID: 27074314 Free PMC article.
-
Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation.J Exp Med. 2018 Jul 2;215(7):1803-1812. doi: 10.1084/jem.20172335. Epub 2018 Jun 18. J Exp Med. 2018. PMID: 29915024 Free PMC article.
-
The Glycosyltransferase ST6Gal-I Protects Tumor Cells against Serum Growth Factor Withdrawal by Enhancing Survival Signaling and Proliferative Potential.J Biol Chem. 2017 Mar 17;292(11):4663-4673. doi: 10.1074/jbc.M116.763862. Epub 2017 Jan 30. J Biol Chem. 2017. PMID: 28154177 Free PMC article.
-
Comprehensive Cell Surface Protein Profiling Identifies Specific Markers of Human Naive and Primed Pluripotent States.Cell Stem Cell. 2017 Jun 1;20(6):874-890.e7. doi: 10.1016/j.stem.2017.02.014. Epub 2017 Mar 23. Cell Stem Cell. 2017. PMID: 28343983 Free PMC article.
-
Glycome profiling by lectin microarray reveals dynamic glycan alterations during epidermal stem cell aging.Aging Cell. 2020 Aug;19(8):e13190. doi: 10.1111/acel.13190. Epub 2020 Jul 18. Aging Cell. 2020. PMID: 32681764 Free PMC article.
References
-
- Jang H. et al.. O-GlcNAc Regulates Pluripotency and Reprogramming by Directly Acting on Core Components of the Pluripotency Network. Cell Stem Cell. 11, 62–74 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

