Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials
- PMID: 26304871
- PMCID: PMC4737863
- DOI: 10.1200/JCO.2015.61.5997
Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials
Abstract
Purpose: The impact of a personalized cancer treatment strategy (ie, matching patients with drugs based on specific biomarkers) is still a matter of debate.
Methods: We reviewed phase II single-agent studies (570 studies; 32,149 patients) published between January 1, 2010, and December 31, 2012 (PubMed search). Response rate (RR), progression-free survival (PFS), and overall survival (OS) were compared for arms that used a personalized strategy versus those that did not.
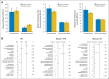
Results: Multivariable analysis (both weighted multiple linear regression and random effects meta-regression) demonstrated that the personalized approach, compared with a nonpersonalized approach, consistently and independently correlated with higher median RR (31% v 10.5%, respectively; P < .001) and prolonged median PFS (5.9 v 2.7 months, respectively; P < .001) and OS (13.7 v 8.9 months, respectively; P < .001). Nonpersonalized targeted arms had poorer outcomes compared with either personalized targeted therapy or cytotoxics, with median RR of 4%, 30%, and 11.9%, respectively; median PFS of 2.6, 6.9, and 3.3 months, respectively (all P < .001); and median OS of 8.7, 15.9, and 9.4 months, respectively (all P < .05). Personalized arms using a genomic biomarker had higher median RR and prolonged median PFS and OS (all P ≤ .05) compared with personalized arms using a protein biomarker. A personalized strategy was associated with a lower treatment-related death rate than a nonpersonalized strategy (median, 1.5% v 2.3%, respectively; P < .001).
Conclusion: Comprehensive analysis of phase II, single-agent arms revealed that, across malignancies, a personalized strategy was an independent predictor of better outcomes and fewer toxic deaths. In addition, nonpersonalized targeted therapies were associated with significantly poorer outcomes than cytotoxic agents, which in turn were worse than personalized targeted therapy.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- Stewart DJ, Kurzrock R. Cancer: The road to Amiens. J Clin Oncol. 2009;27:328–333. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. - PubMed
-
- Dancey JE, Bedard PL, Onetto N, et al. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. - PubMed
-
- Wong KM, Hudson TJ, McPherson JD. Unraveling the genetics of cancer: Genome sequencing and beyond. Annu Rev Genomics Hum Genet. 2011;12:407–430. - PubMed
-
- Barrett JC, Frigault MM, Hollingsworth S, et al. Are companion diagnostics useful? Clin Chem. 2013;59:198–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

