Mitochondrial DNA has a pro-inflammatory role in AMD
- PMID: 26305120
- PMCID: PMC5330253
- DOI: 10.1016/j.bbamcr.2015.08.012
Mitochondrial DNA has a pro-inflammatory role in AMD
Erratum in
-
Corrigendum to "Mitochondrial DNA has a pro-inflammatory role in AMD" [Biochim. Biophys. Acta 1853 (2015) 2897-2906].Biochim Biophys Acta Mol Cell Res. 2024 Dec;1871(8):119790. doi: 10.1016/j.bbamcr.2024.119790. Epub 2024 Jun 29. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38944555 No abstract available.
Abstract
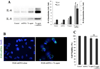
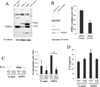
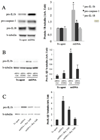
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly of industrialized nations, and there is increasing evidence to support a role for chronic inflammation in its pathogenesis. Mitochondrial DNA (mtDNA) has been recently reported to be pro-inflammatory in various diseases such as Alzheimer's and heart failure. Here, we report that intracellular mtDNA induces ARPE-19 cells to secrete inflammatory cytokines IL-6 and IL-8, which have been consistently associated with AMD onset and progression. The induction was dependent on the size of mtDNA, but not on specific sequence. Oxidative stress plays a major role in the development of AMD, and our findings indicate that mtDNA induces IL-6 and IL-8 more potently when oxidized. Cytokine induction was mediated by STING (Stimulator of Interferon Genes) and NF-κB as evidenced by abrogation of the cytokine response with the use of specific inhibitors (siRNA and BAY 11-7082, respectively). Finally, mtDNA primed the NLRP3 inflammasome. This study contributes to our understanding of the potential pro-inflammatory role of mtDNA in the pathogenesis of AMD.
Keywords: Age-related macular degeneration; Inflammation; Mitochondrial DNA; NLRP3 inflammasome; Retinal pigment epithelium.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

