Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon
- PMID: 26305378
- PMCID: PMC5769816
- DOI: 10.1111/nph.13584
Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon
Abstract
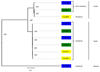
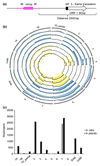
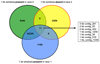
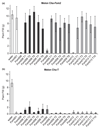
Development of resistant crops is the most effective way to control plant diseases to safeguard food and feed production. Disease resistance is commonly based on resistance genes, which generally mediate the recognition of small proteins secreted by invading pathogens. These proteins secreted by pathogens are called 'avirulence' proteins. Their identification is important for being able to assess the usefulness and durability of resistance genes in agricultural settings. We have used genome sequencing of a set of strains of the melon wilt fungus Fusarium oxysporum f. sp. melonis (Fom), bioinformatics-based genome comparison and genetic transformation of the fungus to identify AVRFOM2, the gene that encodes the avirulence protein recognized by the melon Fom-2 gene. Both an unbiased and a candidate gene approach identified a single candidate for the AVRFOM2 gene. Genetic complementation of AVRFOM2 in three different race 2 isolates resulted in resistance of Fom-2-harbouring melon cultivars. AvrFom2 is a small, secreted protein with two cysteine residues and weak similarity to secreted proteins of other fungi. The identification of AVRFOM2 will not only be helpful to select melon cultivars to avoid melon Fusarium wilt, but also to monitor how quickly a Fom population can adapt to deployment of Fom-2-containing cultivars in the field.
Keywords: AVRFOM2; Fusarium oxysporum f. sp. Melonis; comparative genomics; gene-for-gene interaction; melon Fom-2 resistance gene; virulence gene.
© 2015 The Authors. New Phytologist © 2015 New Phytologist Trust.
Figures

References
-
- Alvarez JM, Gonzalez-Torres R, Mallor C, Gomez-Guillamon ML. Potential sources of resistance to Fusarium wilt and powdery mildew in melons. HortScience. 2005;40:1657–1660.
-
- Appel DJ, Gordon TR. Relationships among pathogenic and nonpathogenic isolates of Fusarium oxysporum based on the partial sequence of the intergenic spacer region of the ribosomal DNA. Molecular Plant–Microbe Interactions. 1996;9:125–138. - PubMed
-
- Brotman Y, Normantovich M, Goldenberg Z, Zvirin Z, Kovalski I, Stovbun N, Doniger T, Bolger AM, Troadec C, Bendahmane A, et al. Dual resistance of melon to Fusarium oxysporum races 0 and 2 and to papaya ring-spot virus is controlled by a pair of head-to-head-oriented NB-LRR genes of unusual architecture. Molecular Plant. 2013;6:235–238. - PubMed
-
- Dangl J, Jones J. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

