A Multiplex Assay for Detection of Staphylococcal and Streptococcal Exotoxins
- PMID: 26305471
- PMCID: PMC4549143
- DOI: 10.1371/journal.pone.0135986
A Multiplex Assay for Detection of Staphylococcal and Streptococcal Exotoxins
Abstract
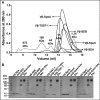
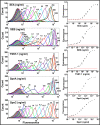
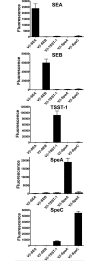
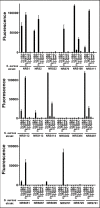
Staphylococcal and streptococcal exotoxins, also known as superantigens, mediate a range of diseases including toxic shock syndrome, and they exacerbate skin, pulmonary and systemic infections caused by these organisms. When present in food sources they can cause enteric effects commonly known as food poisoning. A rapid, sensitive assay for the toxins would enable testing of clinical samples and improve surveillance of food sources. Here we developed a bead-based, two-color flow cytometry assay using single protein domains of the beta chain of T cell receptors engineered for high-affinity for staphylococcal (SEA, SEB and TSST-1) and streptococcal (SpeA and SpeC) toxins. Site-directed biotinylated forms of these high-affinity agents were used together with commercial, polyclonal, anti-toxin reagents to enable specific and sensitive detection with SD50 values of 400 pg/ml (SEA), 3 pg/ml (SEB), 25 pg/ml (TSST-1), 6 ng/ml (SpeA), and 100 pg/ml (SpeC). These sensitivities were in the range of 4- to 80-fold higher than achieved with standard ELISAs using the same reagents. A multiplex format of the assay showed reduced sensitivity due to higher noise associated with the use of multiple polyclonal agents, but the sensitivities were still well within the range necessary for detection in food sources or for rapid detection of toxins in culture supernatants. For example, the assay specifically detected toxins in supernatants derived from cultures of Staphylococcus aureus. Thus, these reagents can be used for simultaneous detection of the toxins in food sources or culture supernatants of potential pathogenic strains of Staphylococcus aureus and Streptococcus pyogenes.
Conflict of interest statement
Figures


References
-
- McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. - PubMed
-
- Evenson ML, Hinds MW, Bernstein RS, Bergdoll MS. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol. 1988;7(4):311–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

