Structural dynamics of E. coli single-stranded DNA binding protein reveal DNA wrapping and unwrapping pathways
- PMID: 26305498
- PMCID: PMC4582245
- DOI: 10.7554/eLife.08193
Structural dynamics of E. coli single-stranded DNA binding protein reveal DNA wrapping and unwrapping pathways
Abstract
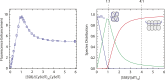
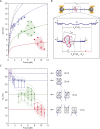
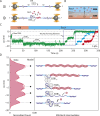
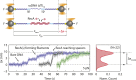
Escherichia coli single-stranded (ss)DNA binding (SSB) protein mediates genome maintenance processes by regulating access to ssDNA. This homotetrameric protein wraps ssDNA in multiple distinct binding modes that may be used selectively in different DNA processes, and whose detailed wrapping topologies remain speculative. Here, we used single-molecule force and fluorescence spectroscopy to investigate E. coli SSB binding to ssDNA. Stretching a single ssDNA-SSB complex reveals discrete states that correlate with known binding modes, the likely ssDNA conformations and diffusion dynamics in each, and the kinetic pathways by which the protein wraps ssDNA and is dissociated. The data allow us to construct an energy landscape for the ssDNA-SSB complex, revealing that unwrapping energy costs increase the more ssDNA is unraveled. Our findings provide insights into the mechanism by which proteins gain access to ssDNA bound by SSB, as demonstrated by experiments in which SSB is displaced by the E. coli recombinase RecA.
Keywords: E. coli; biophysics; energy landscape; optical tweezers; protein-nucleic acid interaction; single molecule; single stranded DNA binding protein; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures














Similar articles
-
Ultrafast redistribution of E. coli SSB along long single-stranded DNA via intersegment transfer.J Mol Biol. 2014 Jun 26;426(13):2413-21. doi: 10.1016/j.jmb.2014.04.023. Epub 2014 May 2. J Mol Biol. 2014. PMID: 24792418 Free PMC article.
-
Imaging and energetics of single SSB-ssDNA molecules reveal intramolecular condensation and insight into RecOR function.Elife. 2015 Sep 18;4:e08646. doi: 10.7554/eLife.08646. Elife. 2015. PMID: 26381353 Free PMC article.
-
Microsecond dynamics of protein-DNA interactions: direct observation of the wrapping/unwrapping kinetics of single-stranded DNA around the E. coli SSB tetramer.J Mol Biol. 2006 May 26;359(1):55-65. doi: 10.1016/j.jmb.2006.02.070. J Mol Biol. 2006. PMID: 16677671
-
The mechanism of action of the SSB interactome reveals it is the first OB-fold family of genome guardians in prokaryotes.Protein Sci. 2021 Sep;30(9):1757-1775. doi: 10.1002/pro.4140. Epub 2021 Jun 14. Protein Sci. 2021. PMID: 34089559 Free PMC article. Review.
-
Molecular insights into the prototypical single-stranded DNA-binding protein from E. coli.Crit Rev Biochem Mol Biol. 2024 Feb-Apr;59(1-2):99-127. doi: 10.1080/10409238.2024.2330372. Epub 2024 May 21. Crit Rev Biochem Mol Biol. 2024. PMID: 38770626 Free PMC article. Review.
Cited by
-
Unravelling How Single-Stranded DNA Binding Protein Coordinates DNA Metabolism Using Single-Molecule Approaches.Int J Mol Sci. 2023 Feb 1;24(3):2806. doi: 10.3390/ijms24032806. Int J Mol Sci. 2023. PMID: 36769124 Free PMC article. Review.
-
Development of a single-stranded DNA-binding protein fluorescent fusion toolbox.Nucleic Acids Res. 2020 Jun 19;48(11):6053-6067. doi: 10.1093/nar/gkaa320. Nucleic Acids Res. 2020. PMID: 32374866 Free PMC article.
-
Observation of long-range tertiary interactions during ligand binding by the TPP riboswitch aptamer.Elife. 2015 Dec 28;4:e12362. doi: 10.7554/eLife.12362. Elife. 2015. PMID: 26709838 Free PMC article.
-
Sodium Ion-Induced Structural Transition on the Surface of a DNA-Interacting Protein.Adv Sci (Weinh). 2024 Nov;11(42):e2401838. doi: 10.1002/advs.202401838. Epub 2024 Sep 20. Adv Sci (Weinh). 2024. PMID: 39301861 Free PMC article.
-
Defining Single Molecular Forces Required for Notch Activation Using Nano Yoyo.Nano Lett. 2016 Jun 8;16(6):3892-7. doi: 10.1021/acs.nanolett.6b01403. Epub 2016 May 12. Nano Lett. 2016. PMID: 27167603 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

