Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial
- PMID: 26305649
- PMCID: PMC5369607
- DOI: 10.1001/jama.2015.9677
Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial
Abstract
Importance: Observational data have suggested that high dietary intake of saturated fat and low intake of vegetables may be associated with increased risk of Alzheimer disease.
Objective: To test the effects of oral supplementation with nutrients on cognitive function.
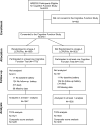
Design, setting, and participants: In a double-masked randomized clinical trial (the Age-Related Eye Disease Study 2 [AREDS2]), retinal specialists in 82 US academic and community medical centers enrolled and observed participants who were at risk for developing late age-related macular degeneration (AMD) from October 2006 to December 2012. In addition to annual eye examinations, several validated cognitive function tests were administered via telephone by trained personnel at baseline and every 2 years during the 5-year study.
Interventions: Long-chain polyunsaturated fatty acids (LCPUFAs) (1 g) and/or lutein (10 mg)/zeaxanthin (2 mg) vs placebo were tested in a factorial design. All participants were also given varying combinations of vitamins C, E, beta carotene, and zinc.
Main outcomes and measures: The main outcome was the yearly change in composite scores determined from a battery of cognitive function tests from baseline. The analyses, which were adjusted for baseline age, sex, race, history of hypertension, education, cognitive score, and depression score, evaluated the differences in the composite score between the treated vs untreated groups. The composite score provided an overall score for the battery, ranging from -22 to 17, with higher scores representing better function.
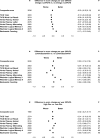
Results: A total of 89% (3741/4203) of AREDS2 participants consented to the ancillary cognitive function study and 93.6% (3501/3741) underwent cognitive function testing. The mean (SD) age of the participants was 72.7 (7.7) years and 57.5% were women. There were no statistically significant differences in change of scores for participants randomized to receive supplements vs those who were not. The yearly change in the composite cognitive function score was -0.19 (99% CI, -0.25 to -0.13) for participants randomized to receive LCPUFAs vs -0.18 (99% CI, -0.24 to -0.12) for those randomized to no LCPUFAs (difference in yearly change, -0.03 [99% CI, -0.20 to 0.13]; P = .63). Similarly, the yearly change in the composite cognitive function score was -0.18 (99% CI, -0.24 to -0.11) for participants randomized to receive lutein/zeaxanthin vs -0.19 (99% CI, -0.25 to -0.13) for those randomized to not receive lutein/zeaxanthin (difference in yearly change, 0.03 [99% CI, -0.14 to 0.19]; P = .66). Analyses were also conducted to assess for potential interactions between LCPUFAs and lutein/zeaxanthin and none were found to be significant.
Conclusions and relevance: Among older persons with AMD, oral supplementation with LCPUFAs or lutein/zeaxanthin had no statistically significant effect on cognitive function.
Trial registration: clinicaltrials.gov Identifier: NCT00345176.
Conflict of interest statement
Emily Y. Chew, Elvira Agrón, and Lenore Launer, are employees of the National Institutes of Health that sponsored the study.
Emily Y. Chew, Elvira Agrón, Traci E. Clemons, Lenore Launer, and Fran Grodstein have no financial or other conflicts of interest.
Paul Bernstein: reported serving as a consultant for Kemin Health, Kalsec, DSM, and Science Based Health.
Figures

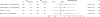
Comment in
-
Lifestyles and Cognitive Health: What Older Individuals Can Do to Optimize Cognitive Outcomes.JAMA. 2015 Aug 25;314(8):774-5. doi: 10.1001/jama.2015.9526. JAMA. 2015. PMID: 26305645 No abstract available.
-
Oral Nutrient Supplementation and Cognitive Function.JAMA. 2016 Feb 2;315(5):515-6. doi: 10.1001/jama.2015.16443. JAMA. 2016. PMID: 26836737 No abstract available.
-
Oral Nutrient Supplementation and Cognitive Function.JAMA. 2016 Feb 2;315(5):516. doi: 10.1001/jama.2015.16452. JAMA. 2016. PMID: 26836738 No abstract available.
-
Oral Nutrient Supplementation and Cognitive Function--Reply.JAMA. 2016 Feb 2;315(5):516-7. doi: 10.1001/jama.2015.16467. JAMA. 2016. PMID: 26836739 Free PMC article. No abstract available.
-
[Supplements for cognitive function unsuitable].MMW Fortschr Med. 2015 Dec 14;157(21-22):52. doi: 10.1007/s15006-015-7616-8. MMW Fortschr Med. 2015. PMID: 26960871 German. No abstract available.
References
-
- 2013 Alzheimer’s disease facts and figures. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9:208–45. - PubMed
-
- Dangour AD, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. The journal of nutrition, health & aging. 2009;13:198–202. - PubMed
-
- Tully AM, Roche HM, Doyle R, et al. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. The British journal of nutrition. 2003;89:483–9. - PubMed
-
- Dangour AD, Allen E, Elbourne D, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. The American journal of clinical nutrition. 2010;91:1725–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

