Performance of a Novel Molecular Method in the Diagnosis of Late-Onset Sepsis in Very Low Birth Weight Infants
- PMID: 26305781
- PMCID: PMC4549273
- DOI: 10.1371/journal.pone.0136472
Performance of a Novel Molecular Method in the Diagnosis of Late-Onset Sepsis in Very Low Birth Weight Infants
Abstract
Objective: To compare the use of a generic molecular assay to 'standard' investigations used to assist the diagnosis of late onset bacterial sepsis in very low birth weight infants (VLBW, <1500 g).
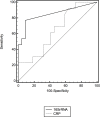
Methods: VLBW infants, greater than 48 hours of age, who were clinically suspected to have sepsis were investigated using standard tests (full blood count, C-reactive protein (at presentation) and blood culture), in addition, blood was taken for a universal molecular assay (16S rRNA reverse transcriptase PCR) for comparison. Clinical data were recorded during the suspected infection episode. A validated sepsis score (NEO-KISS) was used to retrospectively determine the presence of sepsis (independent of blood culture). The performance of each of the tests were compared by sensitivity, specificity, positive/negative likihood ratios (+/-LR) and postive/negative predictive values (PPV/NPV).
Results: Sixty-five babies with suspected clinical sepsis were prospectively included. The performance indicators are presented with 95% confidence limits. For the detection of bacteria, blood culture had sensitivity of 0.57 (0.34-0.78), specificity of 0.45 (0.30-0.61); +LR of 1.05 (0.66-1.66) and-LR of 0.94 (0.52-1.7); PPV of 33.3 (18.56-50.97) and NPV of 68.97 (49.17-87.72). Serum CRP had sensitivity of 0.92 (0.64-1) and specificity of 0.36 (0.17-0.59); +LR of 1.45 (1-2.1) and-LR of 0.21 (0.03-1.5); PPV of 44.46 (26.6-66.6) and NPV of 88.9 (51.8-99.7). The universal molecular assay had sensitivity of 0.76 (0.53-0.92), specificity of 0.95 (0.85-0.99); +LR of 16.8 (4.2-66.3) and-LR of 0.25 (0.1-0.5); PPV of 88.9 (65.3-98.6) and NPV of 89.4 (76.9-96.5).
Conclusions: In VLBW infants this universal molecular assay performed better in the diagnosis of late onset sepsis (LOS) than blood culture and CRP. Further development is required to explore and improve the performance of the assay in real-time diagnosis.
Conflict of interest statement
Figures
References
-
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol 2003;27(4):293–301. - PubMed
-
- Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr 2001;139(6):821–7. - PubMed
-
- Stover BH, Shulman ST, Bratcher DF, Brady MT, Levine GL, Jarvis WR. Nosocomial infection rates in US children’s hospitals' neonatal and pediatric intensive care units. Am J Infect Control 2001;29(3):152–7. - PubMed
-
- Leone CR, Sadeck LS, Vaz FC, Almeida FF. Brazilian Neonatal Research Network(BNRN): Very Low Birth Weight(VLBW) Infant Morbidity and Mortality in Pediatric Acadaemic Society 2327; 2001 Apr 28—May 1. Baltimore MD. USA. Accessed www.abstracts2view.com Feb 2015.
-
- Van der Zwet WC, Kaiser AM, van Elburg RM, Berkhof J, Fetter WPF, Parlevliet GA, et al. Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. J Hosp Infect 2005;61(4):300–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous