Evidence Suggesting a Role of Iron in a Mouse Model of Nephrogenic Systemic Fibrosis
- PMID: 26305890
- PMCID: PMC4549214
- DOI: 10.1371/journal.pone.0136563
Evidence Suggesting a Role of Iron in a Mouse Model of Nephrogenic Systemic Fibrosis
Abstract
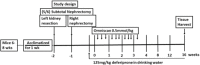
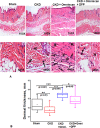
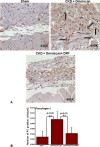
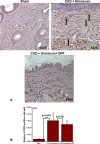
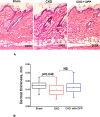
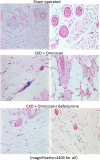
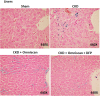
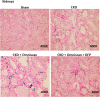
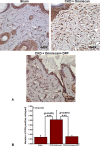
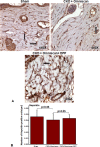
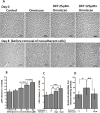
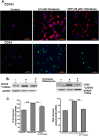
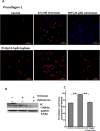
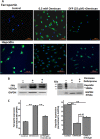
Nephrogenic systemic fibrosis is associated with gadolinium contrast exposure in patients with reduced kidney function and carries high morbidity and mortality. We have previously demonstrated that gadolinium contrast agents induce in vivo systemic iron mobilization and in vitro differentiation of peripheral blood mononuclear cells into ferroportin (iron exporter)-expressing fibrocytic cells. In the present study we examined the role of iron in a mouse model of nephrogenic systemic fibrosis. Chronic kidney disease was induced in 8-week-old male Balb/C mice with a two-step 5/6 nephrectomy surgery. Five groups of mice were studied: control (n = 5), sham surgery control (n = 5), chronic kidney disease control (n = 4), chronic kidney disease injected with 0.5 mmol/kg body weight of Omniscan 3 days per week, for a total of 10 injections (n = 8), and chronic kidney disease with Omniscan plus deferiprone, 125 mg/kg, in drinking water (n = 9). Deferiprone was continued for 16 weeks until the end of the experiment. Mice with chronic kidney disease injected with Omniscan developed skin changes characteristic of nephrogenic systemic fibrosis including hair loss, reddening, ulceration, and skin tightening by 10 to 16 weeks. Histopathological sections demonstrated dermal fibrosis with increased skin thickness (0.25±0.06 mm, sham; 0.34±+0.3 mm, Omniscan-injected). Additionally, we observed an increase in tissue infiltration of ferroportin-expressing, fibrocyte-like cells accompanied by tissue iron accumulation in the skin of the Omniscan-treated mice. The deferiprone-treated group had significantly decreased skin thickness (p<0.05) and significantly decreased dermal fibrosis compared to the Omniscan-only group. In addition, iron chelation prevented tissue infiltration of ferroportin-expressing, fibrocyte-like cells. Our in vitro experiments demonstrated that exposure to Omniscan resulted in the release of catalytic iron and this was prevented by the iron chelator deferiprone. Deferiprone inhibited the differentiation of human peripheral blood mononuclear cells into ferroportin-expressing cells by immunohistochemical staining and western blot analysis. Our studies support an important role of iron in the pathophysiology of gadolinium chelate toxicity and nephrogenic systemic fibrosis.
Conflict of interest statement
Figures

Similar articles
-
Gadolinium contrast agent-induced CD163+ ferroportin+ osteogenic cells in nephrogenic systemic fibrosis.Am J Pathol. 2013 Sep;183(3):796-807. doi: 10.1016/j.ajpath.2013.06.008. Epub 2013 Jul 16. Am J Pathol. 2013. PMID: 23867799 Free PMC article.
-
Effects of gadolinium contrast agents in naïve and nephrectomized rats: relevance to nephrogenic systemic fibrosis.Acta Radiol. 2009 Mar;50(2):156-69. doi: 10.1080/02841850802637808. Acta Radiol. 2009. PMID: 19160079
-
Transmetallation and gadolinium: do low iron stores prevent the development of nephrogenic systemic fibrosis in high-risk end-stage renal disease patients?Hemodial Int. 2010 Jul;14(3):289-94. doi: 10.1111/j.1542-4758.2010.00456.x. Hemodial Int. 2010. PMID: 20662951
-
Nephrogenic systemic fibrosis: a systemic fibrosing disease resulting from gadolinium exposure.Best Pract Res Clin Rheumatol. 2012 Aug;26(4):489-503. doi: 10.1016/j.berh.2012.07.008. Best Pract Res Clin Rheumatol. 2012. PMID: 23040363 Review.
-
Gadolinium toxicity: Iron and ferroportin as central targets.Magn Reson Imaging. 2016 Dec;34(10):1373-1376. doi: 10.1016/j.mri.2016.08.016. Epub 2016 Aug 28. Magn Reson Imaging. 2016. PMID: 27580520 Review.
Cited by
-
A Contrast Examination of Proinflammatory Effects on Kidney Function for γ-Fe2O3 NP and Gadolinium Dimeglumine.Int J Nanomedicine. 2021 Mar 18;16:2271-2282. doi: 10.2147/IJN.S299004. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33776432 Free PMC article.
-
Gadolinium: pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration.Arch Toxicol. 2022 Feb;96(2):403-429. doi: 10.1007/s00204-021-03189-8. Epub 2022 Jan 8. Arch Toxicol. 2022. PMID: 34997254 Free PMC article. Review.
-
Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms.Biometals. 2016 Jun;29(3):365-76. doi: 10.1007/s10534-016-9931-7. Epub 2016 Apr 6. Biometals. 2016. PMID: 27053146 Free PMC article. Review.
-
Gadolinium in Medical Imaging-Usefulness, Toxic Reactions and Possible Countermeasures-A Review.Biomolecules. 2022 May 24;12(6):742. doi: 10.3390/biom12060742. Biomolecules. 2022. PMID: 35740867 Free PMC article. Review.
-
Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents-A Review.Int J Mol Sci. 2024 Apr 6;25(7):4071. doi: 10.3390/ijms25074071. Int J Mol Sci. 2024. PMID: 38612881 Free PMC article. Review.
References
-
- Cowper SE (2008) Nephrogenic systemic fibrosis: an overview. J Amer Coll Radiol 5: 23–28. - PubMed
-
- Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE (2001) Nephrogenic fibrosing dermopathy. Am J Dermatopathol 23: 383–393. - PubMed
-
- Todd DJ, Kagan A, Chibnik LB, Kay J (2007) Cutaneous changes of nephrogenic systemic fibrosis: Predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 56: 3433–3441. - PubMed
-
- Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Hiatt K, et al. (2007) Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. New Engl J Med 357: 720–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

