Design of HIV-1 Protease Inhibitors with Amino-bis-tetrahydrofuran Derivatives as P2-Ligands to Enhance Backbone-Binding Interactions: Synthesis, Biological Evaluation, and Protein-Ligand X-ray Studies
- PMID: 26306007
- PMCID: PMC4765732
- DOI: 10.1021/acs.jmedchem.5b00900
Design of HIV-1 Protease Inhibitors with Amino-bis-tetrahydrofuran Derivatives as P2-Ligands to Enhance Backbone-Binding Interactions: Synthesis, Biological Evaluation, and Protein-Ligand X-ray Studies
Abstract
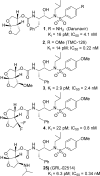
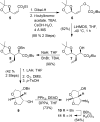
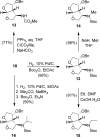
Structure-based design, synthesis, and biological evaluation of a series of very potent HIV-1 protease inhibitors are described. In an effort to improve backbone ligand-binding site interactions, we have incorporated basic-amines at the C4 position of the bis-tetrahydrofuran (bis-THF) ring. We speculated that these substituents would make hydrogen bonding interactions in the flap region of HIV-1 protease. Synthesis of these inhibitors was performed diastereoselectively. A number of inhibitors displayed very potent enzyme inhibitory and antiviral activity. Inhibitors 25f, 25i, and 25j were evaluated against a number of highly-PI-resistant HIV-1 strains, and they exhibited improved antiviral activity over darunavir. Two high resolution X-ray structures of 25f- and 25g-bound HIV-1 protease revealed unique hydrogen bonding interactions with the backbone carbonyl group of Gly48 as well as with the backbone NH of Gly48 in the flap region of the enzyme active site. These ligand-binding site interactions are possibly responsible for their potent activity.
Figures
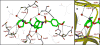
Similar articles
-
Design of gem-difluoro-bis-tetrahydrofuran as P2 ligand for HIV-1 protease inhibitors to improve brain penetration: synthesis, X-ray studies, and biological evaluation.ChemMedChem. 2015 Jan;10(1):107-15. doi: 10.1002/cmdc.201402358. Epub 2014 Oct 21. ChemMedChem. 2015. PMID: 25336073 Free PMC article.
-
Design of substituted tetrahydrofuran derivatives for HIV-1 protease inhibitors: synthesis, biological evaluation, and X-ray structural studies.Org Biomol Chem. 2024 Sep 18;22(36):7354-7372. doi: 10.1039/d4ob00506f. Org Biomol Chem. 2024. PMID: 38973505 Free PMC article.
-
Nonpeptidal P2 ligands for HIV protease inhibitors: structure-based design, synthesis, and biological evaluation.J Med Chem. 1996 Aug 16;39(17):3278-90. doi: 10.1021/jm960128k. J Med Chem. 1996. PMID: 8765511
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Beyond darunavir: recent development of next generation HIV-1 protease inhibitors to combat drug resistance.Chem Commun (Camb). 2022 Oct 20;58(84):11762-11782. doi: 10.1039/d2cc04541a. Chem Commun (Camb). 2022. PMID: 36200462 Free PMC article. Review.
Cited by
-
Structural and binding insights into HIV-1 protease and P2-ligand interactions through molecular dynamics simulations, binding free energy and principal component analysis.J Mol Graph Model. 2019 Nov;92:112-122. doi: 10.1016/j.jmgm.2019.07.008. Epub 2019 Jul 18. J Mol Graph Model. 2019. PMID: 31351319 Free PMC article.
-
Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS.J Med Chem. 2016 Jun 9;59(11):5172-208. doi: 10.1021/acs.jmedchem.5b01697. Epub 2016 Jan 22. J Med Chem. 2016. PMID: 26799988 Free PMC article. Review.
-
Novel Protease Inhibitors Containing C-5-Modified bis-Tetrahydrofuranylurethane and Aminobenzothiazole as P2 and P2' Ligands That Exert Potent Antiviral Activity against Highly Multidrug-Resistant HIV-1 with a High Genetic Barrier against the Emergence of Drug Resistance.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00372-19. doi: 10.1128/AAC.00372-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31085520 Free PMC article.
-
Structural studies of antiviral inhibitor with HIV-1 protease bearing drug resistant substitutions of V32I, I47V and V82I.Biochem Biophys Res Commun. 2019 Jun 30;514(3):974-978. doi: 10.1016/j.bbrc.2019.05.064. Epub 2019 May 12. Biochem Biophys Res Commun. 2019. PMID: 31092330 Free PMC article.
-
Structure-Based Design of Highly Potent HIV-1 Protease Inhibitors Containing New Tricyclic Ring P2-Ligands: Design, Synthesis, Biological, and X-ray Structural Studies.J Med Chem. 2020 May 14;63(9):4867-4879. doi: 10.1021/acs.jmedchem.0c00202. Epub 2020 Apr 29. J Med Chem. 2020. PMID: 32348139 Free PMC article.
References
-
- Mitsuya H, Broder S. Strategies for antiviral therapy in AIDS. Nature. 1987;325:773–778. - PubMed
-
- Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan PR, Hogg RS, Daly P, Kendall P. Association of highly active antiretroviral therapy coverage, population viral load, yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. - PMC - PubMed
-
- Braitstein P, Brinkhof MWG, Dabis F, Schechter M, Boulle A, Miotti P, Wood R, Laurent C, Sprinz E, Seyler C, Bangsberg DR, Balestre E, Sterne JAC, May M, Egger M, Grp A-L, Grp A-C. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. - PubMed
-
- Este JA, Cihlar T. Current status challenges of antiretroviral research therapy. Antiviral Res. 2010;85:25–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

