Mitochondrial Iron-Sulfur Cluster Activity and Cytosolic Iron Regulate Iron Traffic in Saccharomyces cerevisiae
- PMID: 26306041
- PMCID: PMC4646409
- DOI: 10.1074/jbc.M115.676668
Mitochondrial Iron-Sulfur Cluster Activity and Cytosolic Iron Regulate Iron Traffic in Saccharomyces cerevisiae
Abstract
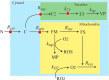
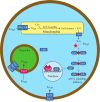
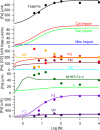
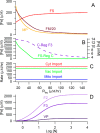
An ordinary differential equation-based mathematical model was developed to describe trafficking and regulation of iron in growing fermenting budding yeast. Accordingly, environmental iron enters the cytosol and moves into mitochondria and vacuoles. Dilution caused by increasing cell volume is included. Four sites are regulated, including those in which iron is imported into the cytosol, mitochondria, and vacuoles, and the site at which vacuolar Fe(II) is oxidized to Fe(III). The objective of this study was to determine whether cytosolic iron (Fecyt) and/or a putative sulfur-based product of iron-sulfur cluster (ISC) activity was/were being sensed in regulation. The model assumes that the matrix of healthy mitochondria is anaerobic, and that in ISC mutants, O2 diffuses into the matrix where it reacts with nonheme high spin Fe(II) ions, oxidizing them to nanoparticles and generating reactive oxygen species. This reactivity causes a further decline in ISC/heme biosynthesis, which ultimately gives rise to the diseased state. The ordinary differential equations that define this model were numerically integrated, and concentrations of each component were plotted versus the concentration of iron in the growth medium and versus the rate of ISC/heme biosynthesis. Model parameters were optimized by fitting simulations to literature data. The model variant that assumed that both Fecyt and ISC biosynthesis activity were sensed in regulation mimicked observed behavior best. Such "dual sensing" probably arises in real cells because regulation involves assembly of an ISC on a cytosolic protein using Fecyt and a sulfur species generated in mitochondria during ISC biosynthesis and exported into the cytosol.
Keywords: Friedreich ataxia; Mossbauer spectroscopy; cellular regulation; mathematical modeling; metal homeostasis.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Mössbauer and LC-ICP-MS investigation of iron trafficking between vacuoles and mitochondria in vma2ΔSaccharomyces cerevisiae.J Biol Chem. 2021 Jan-Jun;296:100141. doi: 10.1074/jbc.RA120.015907. Epub 2020 Dec 6. J Biol Chem. 2021. PMID: 33268384 Free PMC article.
-
Yeast cells depleted of the frataxin homolog Yfh1 redistribute cellular iron: Studies using Mössbauer spectroscopy and mathematical modeling.J Biol Chem. 2022 Jun;298(6):101921. doi: 10.1016/j.jbc.2022.101921. Epub 2022 Apr 10. J Biol Chem. 2022. PMID: 35413285 Free PMC article.
-
A mathematical model of iron import and trafficking in wild-type and Mrs3/4ΔΔ yeast cells.BMC Syst Biol. 2019 Feb 21;13(1):23. doi: 10.1186/s12918-019-0702-2. BMC Syst Biol. 2019. PMID: 30791941 Free PMC article.
-
Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes.Biochim Biophys Acta. 2006 Jul;1763(7):652-67. doi: 10.1016/j.bbamcr.2006.05.011. Epub 2006 May 23. Biochim Biophys Acta. 2006. PMID: 16843540 Review.
-
Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis.Annu Rev Biochem. 2020 Jun 20;89:471-499. doi: 10.1146/annurev-biochem-013118-111540. Epub 2020 Jan 14. Annu Rev Biochem. 2020. PMID: 31935115 Review.
Cited by
-
Mrs4 loss of function in fungi during adaptation to the cystic fibrosis lung.bioRxiv [Preprint]. 2023 Apr 6:2023.04.05.535776. doi: 10.1101/2023.04.05.535776. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0117123. doi: 10.1128/mbio.01171-23. PMID: 37066389 Free PMC article. Updated. Preprint.
-
Iron Homeostatic Regulation in Saccharomyces cerevisiae: Introduction to a Computational Modeling Method.Methods Mol Biol. 2024;2839:3-29. doi: 10.1007/978-1-0716-4043-2_1. Methods Mol Biol. 2024. PMID: 39008245 Free PMC article. Review.
-
Human mitochondrial ferritin exhibits highly unusual iron-O2 chemistry distinct from that of cytosolic ferritins.Nat Commun. 2025 May 20;16(1):4695. doi: 10.1038/s41467-025-59463-1. Nat Commun. 2025. PMID: 40393986 Free PMC article.
-
Ferric ions accumulate in the walls of metabolically inactivating Saccharomyces cerevisiae cells and are reductively mobilized during reactivation.Metallomics. 2016 Jul 13;8(7):692-708. doi: 10.1039/c6mt00070c. Metallomics. 2016. PMID: 27188213 Free PMC article.
-
Mechanisms of iron sensing and regulation in the yeast Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2017 Apr;33(4):75. doi: 10.1007/s11274-017-2215-8. Epub 2017 Mar 17. World J Microbiol Biotechnol. 2017. PMID: 28315258 Review.
References
-
- Chen O. S., Crisp R. J., Valachovic M., Bard M., Winge D. R., and Kaplan J. (2004) Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J. Biol. Chem. 279, 29513–29518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

