An E2 accessory domain increases affinity for the anaphase-promoting complex and ensures E2 competition
- PMID: 26306044
- PMCID: PMC4591839
- DOI: 10.1074/jbc.M115.678193
An E2 accessory domain increases affinity for the anaphase-promoting complex and ensures E2 competition
Abstract
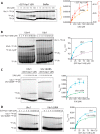
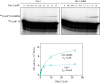
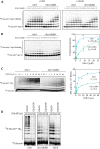
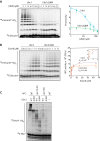
The anaphase-promoting complex/cyclosome (APC/C) is a member of the RING family of E3 ubiquitin ligases, which promote ubiquitin transfer from an E2 ubiquitin-conjugating enzyme to a substrate. In budding yeast, the APC/C collaborates with two E2s, Ubc4 and Ubc1, to promote the initiation and elongation, respectively, of polyubiquitin chains on the substrate. Ubc4 and Ubc1 are thought to compete for the same site on the APC/C, but it is not clear how their affinities are balanced. Here, we demonstrate that a C-terminal ubiquitin-associated (UBA) domain enhances the affinity of Ubc1 for the APC/C. Deletion of the UBA domain reduced apparent APC/C affinity for Ubc1 and decreased polyubiquitin chain length. Surprisingly, the positive effect of the UBA domain was not due to an interaction with the acceptor ubiquitin attached to the APC/C substrate or the donor ubiquitin attached to Ubc1 itself. Instead, our evidence suggests that the UBA domain binds to a site on the APC/C core, thereby increasing Ubc1 affinity and enhancing its ability to compete with Ubc4. The UBA domain is required for normal Ubc1 function and E2 competition in vivo. Thus, the UBA domain of Ubc1 ensures efficient polyubiquitination of substrate by balancing Ubc1 affinity with that of Ubc4.
Keywords: E3 ubiquitin ligase; cell cycle; ubiquitin; ubiquitin-conjugating enzyme (E2 enzyme); yeast.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Sequential E2s drive polyubiquitin chain assembly on APC targets.Cell. 2007 Jul 13;130(1):127-39. doi: 10.1016/j.cell.2007.05.027. Cell. 2007. PMID: 17632060
-
The essential Ubc4/Ubc5 function in yeast is HECT E3-dependent, and RING E3-dependent pathways require only monoubiquitin transfer by Ubc4.J Biol Chem. 2011 Apr 29;286(17):15165-70. doi: 10.1074/jbc.M110.203968. Epub 2011 Feb 25. J Biol Chem. 2011. PMID: 21357418 Free PMC article.
-
The HIP2~ubiquitin conjugate forms a non-compact monomeric thioester during di-ubiquitin synthesis.PLoS One. 2015 Mar 23;10(3):e0120318. doi: 10.1371/journal.pone.0120318. eCollection 2015. PLoS One. 2015. PMID: 25799589 Free PMC article.
-
Orchestra for assembly and fate of polyubiquitin chains.Essays Biochem. 2005;41:1-14. doi: 10.1042/EB0410001. Essays Biochem. 2005. PMID: 16250894 Review.
-
Posing the APC/C E3 Ubiquitin Ligase to Orchestrate Cell Division.Trends Cell Biol. 2019 Feb;29(2):117-134. doi: 10.1016/j.tcb.2018.09.007. Epub 2018 Oct 25. Trends Cell Biol. 2019. PMID: 30482618 Free PMC article. Review.
Cited by
-
Visualizing the complex functions and mechanisms of the anaphase promoting complex/cyclosome (APC/C).Open Biol. 2017 Nov;7(11):170204. doi: 10.1098/rsob.170204. Open Biol. 2017. PMID: 29167309 Free PMC article. Review.
-
From seeds to trees: how E2 enzymes grow ubiquitin chains.Biochem Soc Trans. 2023 Feb 27;51(1):353-362. doi: 10.1042/BST20220880. Biochem Soc Trans. 2023. PMID: 36645006 Free PMC article. Review.
-
The UBA domain of conjugating enzyme Ubc1/Ube2K facilitates assembly of K48/K63-branched ubiquitin chains.EMBO J. 2021 Mar 15;40(6):e106094. doi: 10.15252/embj.2020106094. Epub 2021 Feb 12. EMBO J. 2021. PMID: 33576509 Free PMC article.
-
A comparative study of the cryo-EM structures of Saccharomyces cerevisiae and human anaphase-promoting complex/cyclosome (APC/C).Elife. 2024 Oct 14;13:RP100821. doi: 10.7554/eLife.100821. Elife. 2024. PMID: 39401078 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

