Hsp31 Is a Stress Response Chaperone That Intervenes in the Protein Misfolding Process
- PMID: 26306045
- PMCID: PMC4598993
- DOI: 10.1074/jbc.M115.678367
Hsp31 Is a Stress Response Chaperone That Intervenes in the Protein Misfolding Process
Abstract
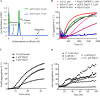
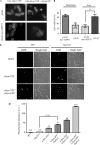
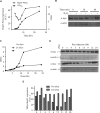
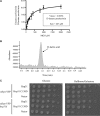
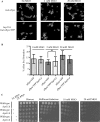
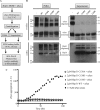
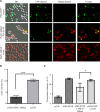
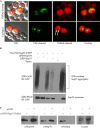
The Saccharomyces cerevisiae heat shock protein Hsp31 is a stress-inducible homodimeric protein that is involved in diauxic shift reprogramming and has glyoxalase activity. We show that substoichiometric concentrations of Hsp31 can abrogate aggregation of a broad array of substrates in vitro. Hsp31 also modulates the aggregation of α-synuclein (αSyn), a target of the chaperone activity of human DJ-1, an Hsp31 homolog. We demonstrate that Hsp31 is able to suppress the in vitro fibrillization or aggregation of αSyn, citrate synthase and insulin. Chaperone activity was also observed in vivo because constitutive overexpression of Hsp31 reduced the incidence of αSyn cytoplasmic foci, and yeast cells were rescued from αSyn-generated proteotoxicity upon Hsp31 overexpression. Moreover, we showed that Hsp31 protein levels are increased by H2O2, in the diauxic phase of normal growth conditions, and in cells under αSyn-mediated proteotoxic stress. We show that Hsp31 chaperone activity and not the methylglyoxalase activity or the autophagy pathway drives the protective effects. We also demonstrate reduced aggregation of the Sup35 prion domain, PrD-Sup35, as visualized by fluorescent protein fusions. In addition, Hsp31 acts on its substrates prior to the formation of large aggregates because Hsp31 does not mutually localize with prion aggregates, and it prevents the formation of detectable in vitro αSyn fibrils. These studies establish that the protective role of Hsp31 against cellular stress is achieved by chaperone activity that intervenes early in the protein misfolding process and is effective on a wide spectrum of substrate proteins, including αSyn and prion proteins.
Keywords: Parkinson disease; alpha-synuclein; enzyme; molecular chaperone; multifunctional protein; prion; protein misfolding; small heat shock protein (sHsp); stress response; yeast.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

