Real-world research and its importance in respiratory medicine
- PMID: 26306101
- PMCID: PMC4487388
- DOI: 10.1183/20734735.015414
Real-world research and its importance in respiratory medicine
Abstract
Educational aims: To improve understanding of: The relative benefits and limitations of evidence derived from different study designs and the role that real-life asthma studies can play in addressing limitations in the classical randomised controlled trial (cRCT) evidence base.The importance of guideline recommendations being modified to fit the populations studied and the model of care provided in their reference studies.
Key points: Classical randomised controlled trials (cRCTs) show results from a narrow patient group with a constrained ecology of care.Patients with "real-life" co-morbidities and lifestyle factors receiving usual care often have different responses to medication which will not be captured by cRCTs if they are excluded by strict selection criteria.Meta-analyses, used to direct guidelines, contain an inherent meta-bias based on patient selection and artificial patient care.Guideline recommendations should clarify where they related to cRCT ideals (in terms of patient populations, medical resources and care received) and could be enhanced through inclusion of evidence from studies designed to better model the populations and care approaches present in routine care.
Summary: Clinical practice requires a complex interplay between experience and training, research, guidelines and judgement, and must not only draw on data from traditional or classical randomised controlled trials (cRCTs), but also from pragmatically designed studies that better reflect real-life clinical practice. To minimise extraneous variables and to optimise their internal validity, cRCTs exclude patients, clinical characteristics and variations in care that could potentially confound outcomes. The result is that respiratory cRCTs often enrol a small, non-representative subset of patients and overlook the important interplay and interactions between patients and the real world, which can effect treatment outcomes. Evidence from real-life studies (e.g. naturalistic or pragmatic clinical trials and observational studies encompassing healthcare database studies and cohort studies) can be combined with cRCT evidence to provide a fuller picture of intervention effectiveness and realistic treatment outcomes, and can provide useful insights into alternative management approaches in more challenging asthma patients. The Respiratory Effectiveness Group (REG), in collaboration with the European Academy of Allergy and Clinical Immunology (EAACI) and the European Respiratory Society (ERS), is developing quality appraisal tools and methods for integrating different sources of evidence. A REG/EAACI taskforce aims to help support future guideline developers to avoid a one-size-fits-all approach to recommendations and to tailor the conclusions of their meta-analyses to the populations under consideration.
Conflict of interest statement
Figures
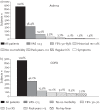
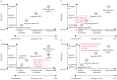
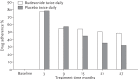


References
-
- Wong GWK, Miravitlles M, Chisholm A, et al. . Respiratory guidelines—ATS annals. Ann Am Thorac Soc 2014; 11(Suppl. 2): S83–S84. - PubMed
-
- Schünemann HJ, Jaeschke R, Cook DJ, et al. . An Official ATS Statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006; 174: 605–614. - PubMed
-
- World Health Organization (WHO). Annex 1. GRADE Evidence Profiles. http://www.who.int/hrh/retention/annex1_grade_evidence_profiles.pdf. Date last accessed: December 24, 2014. Date updated: not stated.
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. http://handbook.cochrane.org/chapter_12/12_2_1_the_grade_approach.htm. Date last accessed: December 24, 2014. Date updated: not stated.
-
- Herland K, Akselsen JP, Skjonsberg OH, et al. . How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med 2005; 99: 11–19. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
