Optimising inhaled mannitol for cystic fibrosis in an adult population
- PMID: 26306102
- PMCID: PMC4487380
- DOI: 10.1183/20734735.021414
Optimising inhaled mannitol for cystic fibrosis in an adult population
Abstract
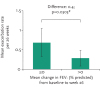
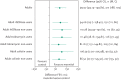
Abstract: There has been remarkable progress in the treatment of cystic fibrosis (CF) patients over the past 20 years. However, limitations of standard therapies have highlighted the need for a convenient alternative treatment to effectively target the pathophysiologic basis of CF-related disease by improving mucociliary clearance of airway secretions and consequently improve lung function and reduce respiratory exacerbations. Mannitol is an osmotic agent available as a dry powder, dispensed in a convenient disposable inhaler device for the treatment of adult patients with CF. Inhalation of mannitol as a dry powder is thought to change the viscoelastic properties of airway secretions, increase the hydration of the airway surface liquid and contribute to increased mucociliary and cough clearance of retained secretions. In two large phase 3 studies [1, 2], long-term use of inhaled mannitol resulted in a significant and clinically meaningful improvement in lung function relative to control in adult CF subjects and had an acceptable safety profile. Clinical experience with inhaled mannitol confirms that it is safe and effective. A minority of patients are unable to tolerate the medication. However, through training in proper inhaler technique and setting clear expectations regarding therapeutic effects, both the tolerance and adherence necessary for long term efficacy can be positively influenced.
Educational aims: To discuss the importance of airway clearance treatments in the management of cystic fibrosis.To describe the clinical data that supports the use of mannitol in adult patients with cystic fibrosis.To highlight the role of mannitol tolerance testing in screening for hyperresponsiveness.To provide practical considerations for patient education in use of mannitol inhaler.
Key points: Inhaled mannitol is a safe and effective option in adult patients with cystic fibrosis.Mannitol tolerance testing effectively screens for hyperresponsiveness prior to initiation of therapy.Physiotherapists and respiratory therapists play an integral role in the introduction and maintenance of dry powder inhalation therapy.Patient training and follow-up is important for optimising longer term adherence.
Figures

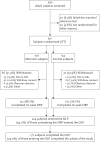
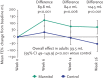
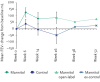
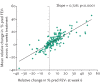
References
-
- Bilton D, Robinson P, Cooper P, et al. . Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J 2011; 38: 1071–1080. - PubMed
-
- Aitken ML, Bellon G, De Boeck K, et al. . Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med 2012; 185: 645–652. - PubMed
-
- Flume PA. Pulmonary complications of cystic fibrosis. Respir Care 2009; 54: 618–627. - PubMed
-
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Int Med 2007; 261: 5–16. - PubMed
-
- Flume PA, Robinson KA, O’Sullivan BP, et al. . Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009; 54: 522–537. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
