Granular Quality Reporting for Cervical Cytology Testing
- PMID: 26306264
- PMCID: PMC4525216
Granular Quality Reporting for Cervical Cytology Testing
Abstract
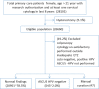
Quality reporting for cervical cancer prevention is focused on patients with normal cervical cytology, and excludes patients with cytological abnormalities that may be at higher risk. The major obstacles for granular reporting are the complexity of surveillance guidelines and free-text data. We performed automated chart review to compare the cytology testing rates for patients with 'atypical squamous cells of undetermined significance' (ASCUS) cytology, with the rates for patients with normal cytology. We modeled the surveillance guidelines, and extracted information from free-text cytology reports, to perform this study on 28101 female patients. Our results show that patients with ASCUS cytology had significantly higher adherence rates (94.9%) than those for patients with normal cytology (90.4%). Overall our study indicates that the quality of care varies significantly between the high and average risk patients. Our study demonstrates the use of health information technology for higher granularity of reporting for cervical cytology testing.
Figures
References
-
- ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012 Nov;120(5):1222–38. - PubMed
-
- Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2006 2007 Oct;197(4):346–55. - PubMed
-
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012 Apr;137(4):516–42. - PubMed
-
- Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jun 19;156(12):880–91. W312. - PubMed
-
- Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013 Apr;17(5 Suppl 1):S1–S27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources