PD-L1 Monoclonal Antibody Treats Ischemic Stroke by Controlling Central Nervous System Inflammation
- PMID: 26306753
- PMCID: PMC4589506
- DOI: 10.1161/STROKEAHA.115.010592
PD-L1 Monoclonal Antibody Treats Ischemic Stroke by Controlling Central Nervous System Inflammation
Abstract
Background and purpose: Both pathogenic and regulatory immune processes are involved in the middle cerebral artery occlusion (MCAO) model of experimental stroke, including interactions involving the programmed death 1 (PD-1) receptor and its 2 ligands, PD-L1 and PD-L2. Although PD-1 reduced stroke severity, PD-L1 and PD-L2 appeared to play pathogenic roles, suggesting the use of anti-PD-L monoclonal antibody therapy for MCAO.
Methods: Male C57BL/6 mice were treated with a single dose of anti-PD-L1 monoclonal antibody 4 hours after MCAO and evaluated for clinical, histological and immunologic changes after 96 hours of reperfusion.
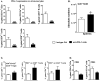
Results: Blockade of the PD-L1 checkpoint using a single injection of 200 μg anti-PD-L1 monoclonal antibody given intravenously 4 hours after occlusion significantly reduced MCAO infarct volumes and improved neurological outcomes after 96 hours of reperfusion. Treatment partially reversed splenic atrophy and decreased central nervous system infiltrating immune cells concomitant with enhanced appearance of CD8(+) regulatory T cells in the lesioned central nervous system hemisphere.
Conclusions: This study demonstrates for the first time the beneficial therapeutic effects of PD-L1 checkpoint blockade on MCAO, thus validating proposed mechanisms obtained in our previous studies using PD-1- and PD-L-deficient mice. These results provide strong support for the use of available humanized anti-PD-L1 antibodies for treatment of human stroke subjects.
Keywords: anti-PD-L1 antibody therapy; interleukin-10; middle cerebral artery occlusion; reperfusion; stroke.
© 2015 American Heart Association, Inc.
Figures
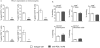
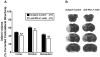

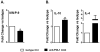
References
-
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. - PubMed
-
- Schaller B, Graf R. Cerebral ischemia and reperfusion: The pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–371. - PubMed
-
- Macrez R, Ali C, Toutirais O, LeMauff B, Defer G, Dirnagle U, et al. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

