Proteomics of the Synapse--A Quantitative Approach to Neuronal Plasticity
- PMID: 26307175
- PMCID: PMC4739661
- DOI: 10.1074/mcp.R115.051482
Proteomics of the Synapse--A Quantitative Approach to Neuronal Plasticity
Abstract
The advances in mass spectrometry based proteomics in the past 15 years have contributed to a deeper appreciation of protein networks and the composition of functional synaptic protein complexes. However, research on protein dynamics underlying core mechanisms of synaptic plasticity in brain lag far behind. In this review, we provide a synopsis on proteomic research addressing various aspects of synaptic function. We discuss the major topics in the study of protein dynamics of the chemical synapse and the limitations of current methodology. We highlight recent developments and the future importance of multidimensional proteomics and metabolic labeling. Finally, emphasis is given on the conceptual framework of modern proteomics and its current shortcomings in the quest to gain a deeper understanding of synaptic plasticity.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
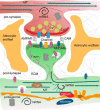

References
-
- Satoh K., Takeuchi M., Oda Y., Deguchi-Tawarada M., Sakamoto Y., Matsubara K., Nagasu T., and Takai Y. (2002) Identification of activity-regulated proteins in the postsynaptic density fraction. Genes Cells 7, 187–197 - PubMed
-
- Jordan B. A., Fernholz B. D., Boussac M., Xu C., Grigorean G., Ziff E. B., and Neubert T. A. (2004) Identification and verification of novel rodent postsynaptic density proteins. Mol. Cell. Proteomics 3, 857–871 - PubMed
-
- Li K. W., Hornshaw M. P., Van Der Schors R. C., Watson R., Tate S., Casetta B., Jimenez C. R., Gouwenberg Y., Gundelfinger E. D., Smalla K. H., and Smit A. B. (2004) Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology. J. Biol. Chem. 27, 987–1002 - PubMed
-
- Li K., Hornshaw M. P., Van Minnen J., Smalla K. H., Gundelfinger E. D., and Smit A. B. (2005) Organelle proteomics of rat synaptic proteins: correlation-profiling by isotope-coded affinity tagging in conjunction with liquid chromatography-tandem mass spectrometry to reveal postsynaptic density specific proteins. J. Proteome Res. 4, 725–733 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

