An assessment of sex bias in neurodevelopmental disorders
- PMID: 26307204
- PMCID: PMC4549901
- DOI: 10.1186/s13073-015-0216-5
An assessment of sex bias in neurodevelopmental disorders
Abstract
Background: Neurodevelopmental disorders such as autism and intellectual disability have a sex bias skewed towards boys; however, systematic assessment of this bias is complicated by the presence of significant genetic and phenotypic heterogeneity of these disorders.
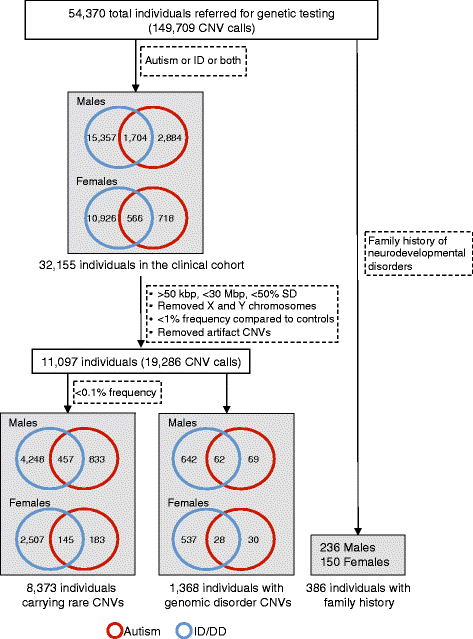
Methods: To assess the extent and characteristics of sex bias, we analyzed the frequency of comorbid features, the magnitude of genetic load, and the existence of family history within 32,155 individuals ascertained clinically for autism or intellectual disability/developmental delay (ID/DD), including a subset of 8,373 individuals carrying rare copy-number variants (CNVs).
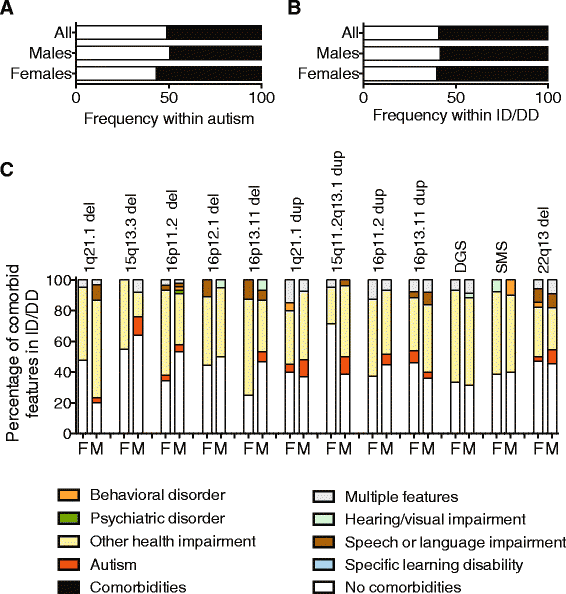
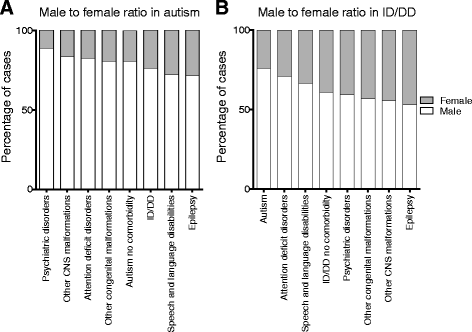
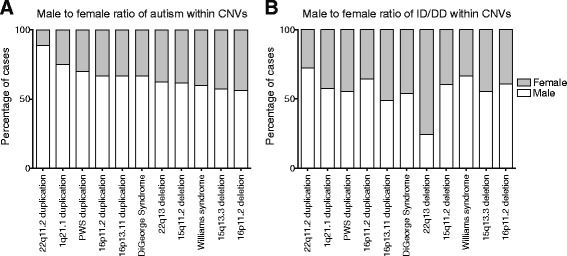
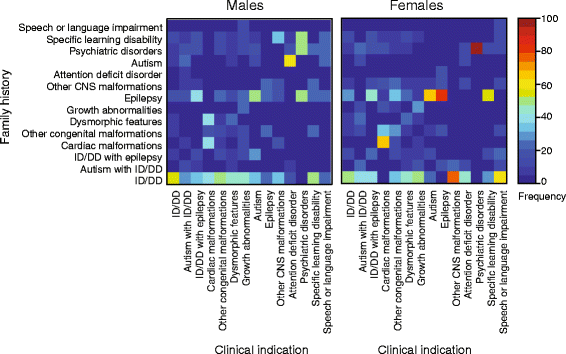
Results: We find that girls were more likely than boys to show comorbid features within both autism (P = 2.9 × 10(-6), OR = 1.34) and ID/DD (P = 7.2 × 10(-4), OR = 1.08) cohorts. The frequency of comorbid features in ID/DD was higher in boys (1q21.1 deletion, 15q11.2q13.1 duplication) or girls (15q13.3 deletion, 16p11.2 deletion) carrying specific CNVs associated with variable expressivity while such differences were the smallest for syndromic CNVs (Smith-Magenis syndrome, DiGeorge syndrome). The extent of the male sex bias also varied according to the specific comorbid feature, being most extreme for autism with psychiatric comorbidities and least extreme for autism comorbid with epilepsy. The sex ratio was also specific to certain CNVs, from an 8:1 male:female ratio observed among autistic individuals carrying the 22q11.2 duplication to 1.3:1 male:female ratio in those carrying the 16p11.2 deletion. Girls carried a higher burden of large CNVs compared to boys for autism or ID/DD, and this difference diminished when severe comorbidities were considered. Affected boys showed a higher frequency of neuropsychiatric family histories such as autism (P = 0.01) or specific learning disability (P = 0.03), while affected girls showed a higher frequency of developmental family histories such as growth abnormalities (P = 0.02).
Conclusions: The sex bias within neurodevelopmental disorders is influenced by the presence of specific comorbidities, specific CNVs, mutational burden, and pre-existing family history of neurodevelopmental phenotypes.
Figures

References
-
- Autism and Developmental Disabilities Monitoring Network Surveillance Year Principal Investigators, Centers for Disease Control and Prevention Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

