Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice
- PMID: 26307667
- PMCID: PMC4754541
- DOI: 10.1038/mt.2015.150
Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice
Abstract
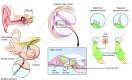
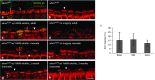
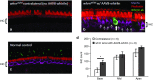
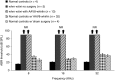
Hereditary deafness is one of the most common disabilities affecting newborns. Many forms of hereditary deafness are caused by morphological defects of the stereocilia bundles on the apical surfaces of inner ear hair cells, which are responsible for sound detection. We explored the effectiveness of gene therapy in restoring the hair cell stereocilia architecture in the whirlin mouse model of human deafness, which is deaf due to dysmorphic, short stereocilia. Wild-type whirlin cDNA was delivered via adeno-associated virus (AAV8) by injection through the round window of the cochleas in neonatal whirler mice. Subsequently, whirlin expression was detected in infected hair cells (IHCs), and normal stereocilia length and bundle architecture were restored. Whirlin gene therapy also increased inner hair cell survival in the treated ears compared to the contralateral nontreated ears. These results indicate that a form of inherited deafness due to structural defects in cochlear hair cells is amenable to restoration through gene therapy.
Figures



References
-
- Morton, NE (1991). Genetic epidemiology of hearing impairment. Ann N Y Acad Sci 630: 16–31. - PubMed
-
- Lenz, DR and Avraham, KB (2011). Hereditary hearing loss: from human mutation to mechanism. Hear Res 281: 3–10. - PubMed
-
- Richardson, GP, de Monvel, JB and Petit, C (2011). How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol 73: 311–334. - PubMed
-
- Belyantseva, IA, Labay, V, Boger, ET, Griffith, AJ and Friedman, TB (2003). Stereocilia: the long and the short of it. Trends Mol Med 9: 458–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

