A caveolin-dependent and PI3K/AKT-independent role of PTEN in β-catenin transcriptional activity
- PMID: 26307673
- PMCID: PMC4560817
- DOI: 10.1038/ncomms9093
A caveolin-dependent and PI3K/AKT-independent role of PTEN in β-catenin transcriptional activity
Abstract
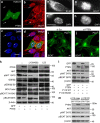
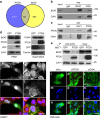
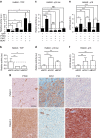
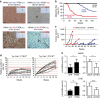
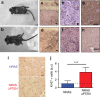
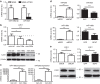
Loss of the tumour suppressor PTEN is frequent in human melanoma, results in MAPK activation, suppresses senescence and mediates metastatic behaviour. How PTEN loss mediates these effects is unknown. Here we show that loss of PTEN in epithelial and melanocytic cell lines induces the nuclear localization and transcriptional activation of β-catenin independent of the PI3K-AKT-GSK3β axis. The absence of PTEN leads to caveolin-1 (CAV1)-dependent β-catenin transcriptional modulation in vitro, cooperates with NRAS(Q61K) to initiate melanomagenesis in vivo and induces efficient metastasis formation associated with E-cadherin internalization. The CAV1-β-catenin axis is mediated by a feedback loop in which β-catenin represses transcription of miR-199a-5p and miR-203, which suppress the levels of CAV1 mRNA in melanoma cells. These data reveal a mechanism by which loss of PTEN increases CAV1-mediated dissociation of β-catenin from membranous E-cadherin, which may promote senescence bypass and metastasis.
Figures


References
-
- Dhomen N. et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 (2009). - PubMed
-
- Michaloglou C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005). - PubMed
-
- Ackermann J. et al. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 65, 4005–4011 (2005). - PubMed
-
- Shakhova O. et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat. Cell Biol. 14, 882–890 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

