Melanin Transfer in Human 3D Skin Equivalents Generated Exclusively from Induced Pluripotent Stem Cells
- PMID: 26308443
- PMCID: PMC4550351
- DOI: 10.1371/journal.pone.0136713
Melanin Transfer in Human 3D Skin Equivalents Generated Exclusively from Induced Pluripotent Stem Cells
Abstract
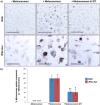
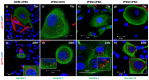
The current utility of 3D skin equivalents is limited by the fact that existing models fail to recapitulate the cellular complexity of human skin. They often contain few cell types and no appendages, in part because many cells found in the skin are difficult to isolate from intact tissue and cannot be expanded in culture. Induced pluripotent stem cells (iPSCs) present an avenue by which we can overcome this issue due to their ability to be differentiated into multiple cell types in the body and their unlimited growth potential. We previously reported generation of the first human 3D skin equivalents from iPSC-derived fibroblasts and iPSC-derived keratinocytes, demonstrating that iPSCs can provide a foundation for modeling a complex human organ such as skin. Here, we have increased the complexity of this model by including additional iPSC-derived melanocytes. Epidermal melanocytes, which are largely responsible for skin pigmentation, represent the second most numerous cell type found in normal human epidermis and as such represent a logical next addition. We report efficient melanin production from iPSC-derived melanocytes and transfer within an entirely iPSC-derived epidermal-melanin unit and generation of the first functional human 3D skin equivalents made from iPSC-derived fibroblasts, keratinocytes and melanocytes.
Conflict of interest statement
Figures



References
-
- Cantón I, Sarwar U, Kemp EH, Ryan AJ, MacNeil S, Haycock JW. Real-time detection of stress in 3D tissue-engineered constructs using NF-kappaB activation in transiently transfected human dermal fibroblast cells. Tissue Eng. 2007;13: 1013–24. - PubMed
-
- Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424: 870–872. - PubMed
-
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5: 675–688. - PubMed
-
- Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69–70: 81–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

