Exploring the population-level impact of MenB vaccination via modeling: Potential for serogroup replacement
- PMID: 26308796
- PMCID: PMC5049729
- DOI: 10.1080/21645515.2015.1080400
Exploring the population-level impact of MenB vaccination via modeling: Potential for serogroup replacement
Abstract
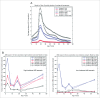
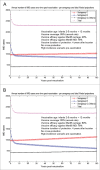
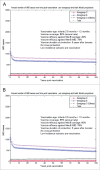
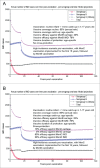
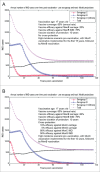
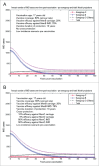
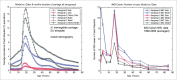
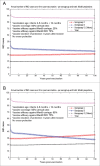
Various meningococcal conjugate vaccines exist against serogroups A, C, W and Y. A new protein-based vaccine targeting serogroup B (MenB) is also now available. The potential of such vaccines to drive serogroup replacement is considered a possible public health concern when implementing nationwide routine immunization programmes. The aim of this work was to investigate if and how serogroup replacement may occur following widespread vaccination with a MenB vaccine that may protect against carriage. To that end, we built a dynamic transmission model with age and serogroup stratification, focusing on European settings where most invasive meningococcal disease (IMD) cases are caused by serogroups B and C. For illustration purposes, the model was employed in 2 such settings: UK (England and Wales) and Czech Republic. Preliminary model-based projections suggest that, under strong serogroup competition for colonization, vaccine-induced serogroup replacement may occur even with a relatively low vaccine efficacy against serogroup B carriage (e.g., 20%), with potential subsequent increase in serogroup C IMD. The magnitude and speed of the model-projected serogroup C IMD increase depend on the MenB vaccination strategy, vaccine efficacy against carriage and the extent of any potential cross-protection against other serogroups. These analyses are neither exhaustive nor definitive, and focused on simulating potential population-level trends in IMD post-vaccination, under certain assumptions. Due to present inherent limitations and uncertainties, this study has limited quantitative value and is best regarded as an explorative qualitative modeling approach, to complement and challenge the current status quo, and suggest areas where collecting additional data may be essential.
Keywords: dynamic transmission model; invasive meningococcal disease; mathematical modeling; serogroup B meningococcal vaccine; serogroup replacement.
Figures




References
-
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853-61; PMID:21075057; http://dx.doi.org/10.1016/S1473-3099(10)70251-6 - DOI - PubMed
-
- ECDC, 2013 Annual epidemiological report Reporting on 2011 surveillance data and 2012 epidemic intelligence data. Available at: http://www.ecdc.europa.eu/en/publications/Publications/Annual-Epidemiolo... [Accessed 10 February 2015].
-
- Soriano-Gabarro M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther 2011; 9:761-74; PMID:21905785; http://dx.doi.org/10.1586/eri.11.89 - DOI - PubMed
-
- Zahlanie YC, Hammadi MM, Ghanem ST, Dbaibo GS. Review of meningococcal vaccines with updates on immunization in adults. Hum Vaccin Immunother 2014; 10:995-1007; PMID:24500529; http://dx.doi.org/10.4161/hv.27739 - DOI - PMC - PubMed
-
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1-28; PMID:23515099 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
