Epithelial Sodium Channel-Mediated Sodium Transport Is Not Dependent on the Membrane-Bound Serine Protease CAP2/Tmprss4
- PMID: 26309024
- PMCID: PMC4550455
- DOI: 10.1371/journal.pone.0135224
Epithelial Sodium Channel-Mediated Sodium Transport Is Not Dependent on the Membrane-Bound Serine Protease CAP2/Tmprss4
Abstract
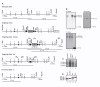
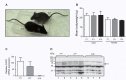
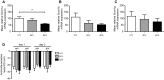
The membrane-bound serine protease CAP2/Tmprss4 has been previously identified in vitro as a positive regulator of the epithelial sodium channel (ENaC). To study its in vivo implication in ENaC-mediated sodium absorption, we generated a knockout mouse model for CAP2/Tmprss4. Mice deficient in CAP2/Tmprss4 were viable, fertile, and did not show any obvious histological abnormalities. Unexpectedly, when challenged with sodium-deficient diet, these mice did not develop any impairment in renal sodium handling as evidenced by normal plasma and urinary sodium and potassium electrolytes, as well as normal aldosterone levels. Despite minor alterations in ENaC mRNA expression, we found no evidence for altered proteolytic cleavage of ENaC subunits. In consequence, ENaC activity, as monitored by the amiloride-sensitive rectal potential difference (ΔPD), was not altered even under dietary sodium restriction. In summary, ENaC-mediated sodium balance is not affected by lack of CAP2/Tmprss4 expression and thus, does not seem to directly control ENaC expression and activity in vivo.
Conflict of interest statement
Figures




Similar articles
-
ENaC activation by proteases.Acta Physiol (Oxf). 2022 May;235(1):e13811. doi: 10.1111/apha.13811. Epub 2022 Mar 21. Acta Physiol (Oxf). 2022. PMID: 35276025 Free PMC article. Review.
-
ENaC proteolytic regulation by channel-activating protease 2.J Gen Physiol. 2008 Nov;132(5):521-35. doi: 10.1085/jgp.200810030. Epub 2008 Oct 13. J Gen Physiol. 2008. PMID: 18852303 Free PMC article.
-
In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases.Am J Physiol Lung Cell Mol Physiol. 2005 Jun;288(6):L1099-109. doi: 10.1152/ajplung.00332.2004. Epub 2005 Jan 28. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15681398
-
Deletion of the serine protease CAP2/Tmprss4 leads to dysregulated renal water handling upon dietary potassium depletion.Sci Rep. 2019 Dec 20;9(1):19540. doi: 10.1038/s41598-019-55995-x. Sci Rep. 2019. PMID: 31863073 Free PMC article.
-
Regulation of the epithelial Na+ channel by peptidases.Curr Top Dev Biol. 2007;78:23-46. doi: 10.1016/S0070-2153(06)78002-4. Curr Top Dev Biol. 2007. PMID: 17338914 Free PMC article. Review.
Cited by
-
Membrane-anchored serine proteases as regulators of epithelial function.Biochem Soc Trans. 2020 Apr 29;48(2):517-528. doi: 10.1042/BST20190675. Biochem Soc Trans. 2020. PMID: 32196551 Free PMC article. Review.
-
The epithelial Na+ channel α- and γ-subunits are cleaved at predicted furin-cleavage sites, glycosylated and membrane associated in human kidney.Pflugers Arch. 2019 Dec;471(11-12):1383-1396. doi: 10.1007/s00424-019-02321-z. Epub 2019 Nov 21. Pflugers Arch. 2019. PMID: 31654198
-
Cell surface-anchored serine proteases in cancer progression and metastasis.Cancer Metastasis Rev. 2019 Sep;38(3):357-387. doi: 10.1007/s10555-019-09811-7. Cancer Metastasis Rev. 2019. PMID: 31529338 Free PMC article. Review.
-
ENaC activation by proteases.Acta Physiol (Oxf). 2022 May;235(1):e13811. doi: 10.1111/apha.13811. Epub 2022 Mar 21. Acta Physiol (Oxf). 2022. PMID: 35276025 Free PMC article. Review.
-
The Proteolytic Activation of (H3N2) Influenza A Virus Hemagglutinin Is Facilitated by Different Type II Transmembrane Serine Proteases.J Virol. 2016 Apr 14;90(9):4298-4307. doi: 10.1128/JVI.02693-15. Print 2016 May. J Virol. 2016. PMID: 26889029 Free PMC article.
References
-
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 1993;361: 467–470. - PubMed
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994;367: 463–467. - PubMed
-
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 1997;389: 607–610. - PubMed
-
- Vallet V, Horisberger JD, Rossier BC. Epithelial sodium channel regulatory proteins identified by function expression cloning. Kidney Int. 1998;54: 109–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

