Detection of fetal cell-free DNA in maternal plasma for Down syndrome, Edward syndrome and Patau syndrome of high risk fetus
- PMID: 26309618
- PMCID: PMC4538030
Detection of fetal cell-free DNA in maternal plasma for Down syndrome, Edward syndrome and Patau syndrome of high risk fetus
Abstract
Objective: The study aimed to validate the efficacy of detection of fetal cell-free DNA in maternal plasma of trisomy 21, 18 and 13 in a clinical setting.
Methods: A total of 2340 women at high risk for Down syndrome based on maternal age, prenatal history or a positive sesum or sonographic screening test were offered prenatal noninvasive aneuploidy test. According to the prenatal noninvasive aneuploidy test, the pregnant women at high risk were offered amniocentesis karyotype analysis and the pregnant at low risk were followed up to make sure the newborn outcome.
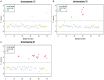
Results: The prenatal noninvasive aneuploidy test was positive for trisomy 21 in 17 cases, for trisomy 18 in 6 cases and for trisomy 13 in 1 case, which of all were confirmed by karyotype analysis. Newborns of low risk gestational woman detected by prenatal noninvasive aneuploidy for trisomy 21, 18, 13 were followed up and no one was found with trisomy.
Conclusions: The prenatal noninvasive aneuploidy test is highly accurate for detection of trisomy 21, 18 and 13, which can be considered as a practical alternative for traditional invasive diagnostic procedures.
Keywords: Down syndrome; fetal cell-free DNA; prenatal noninvasive diagnosis.
Figures

References
-
- Boormans EM, Birnie E, Wildschut HI, Schuring-Blom HG, Oepkes D, van Oppen CA, Nijhuis JG, Macville MV, Kooper AJ, Huijsdens K, Hoffer MV, Go A, Creemers J, Bhola SL, Bilardo KM, Suijkerbuijk R, Bouman K, Galjaard RJ, Bonsel GJ, van Lith JM. Multiplex ligation-dependent probe amplification versus karyotyping in prenatal diagnosis: the M. A.K.E. Study. BMC Pregnancy Childbirth. 2008;8:18. - PMC - PubMed
-
- Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890–901. - PubMed
-
- Sparks AB, Wang ET, Struble CA, Barrett W, Stokowski R, McBride C, Zahn J, Lee K, Shen N, Doshi J, Sun M, Garrison J, Sandler J, Hollemon D, Pattee P, Tomita-Mitchell A, Mitchell M, Stuelpnagel J, Song K, Oliphant A. Selective analysis of cell-free DNA in maternal blood for evaluation of fetal trisomy. Prenat Diagn. 2012;32:3–9. - PMC - PubMed
-
- Weisz B, Pandya P, Chitty L, Jones P, Huttly W, Rodeck C. Practical issues drawn from the implementation of the integrated test for Down syndrome screening into routine clinical practice. BJOG. 2007;114:493–497. - PubMed
LinkOut - more resources
Full Text Sources
