Treating Proximal Tibial Growth Plate Injuries Using Poly(Lactic-co-Glycolic Acid) Scaffolds
- PMID: 26309783
- PMCID: PMC4497685
- DOI: 10.1089/biores.2014.0034
Treating Proximal Tibial Growth Plate Injuries Using Poly(Lactic-co-Glycolic Acid) Scaffolds
Abstract
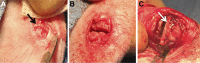
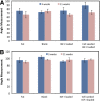
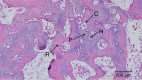
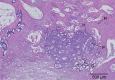
Growth plate fractures account for nearly 18.5% of fractures in children. Depending on the type and severity of the injury, inhibited bone growth or angular deformity caused by bone forming in place of the growth plate can occur. The current treatment involves removal of the bony bar and replacing it with a filler substance, such as a free fat graft. Unfortunately, reformation of the bony bar frequently occurs, preventing the native growth plate from regenerating. The goal of this pilot study was to determine whether biodegradable scaffolds can enhance native growth plate regeneration following a simulated injury that resulted in bony bar formation in the proximal tibial growth plate of New Zealand white rabbits. After removing the bony bar, animals received one of the following treatments: porous poly(lactic-co-glycolic acid) (PLGA) scaffold; PLGA scaffold loaded with insulin-like growth factor I (IGF-I); PLGA scaffold loaded with IGF-I and seeded with autogenous bone marrow cells (BMCs) harvested at the time of implantation; or fat graft (as used clinically). The PLGA scaffold group showed an increased chondrocyte population and a reduced loss of the remaining native growth plate compared to the fat graft group (the control group). An additional increase in chondrocyte density was seen in scaffolds loaded with IGF-I, and even more so when BMCs were seeded on the scaffold. While there was no significant reduction in the angular deformation of the limbs, the PLGA scaffolds increased the amount of cartilage and reduced the amount of bony bar reformation.
Keywords: growth plate; insulin-like growth factor I; physeal injury; poly(lactic-co-glycolic acid); scaffold.
Figures






References
-
- Iannotti J. Growth plate physiology and pathology. Orthop Clin North Am. 1990;21:1. - PubMed
-
- Burdan F, Szumiło J, Korobowicz A, et al. . Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol. 2009;47:5–16 - PubMed
-
- Ballock RT, O'Keefe RJ. The biology of the growth plate. J Bone Joint Surg. 2003;85:715–726 - PubMed
-
- Mizuta T, Benson W, Foster B, Morris L. Statistical analysis of the incidence of physeal injuries. J Pediatr Orthoped. 1987;7:518–523 - PubMed
-
- Basener CJ, Mehlman CT, DiPasquale TG. Growth disturbance after distal femoral growth plate fractures in children: a meta-analysis. J Orthop Trauma. 2009;23:663–667 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
