Subcutaneous Adipose Tissue-Derived Stem Cell Utility Is Independent of Anatomical Harvest Site
- PMID: 26309790
- PMCID: PMC4497709
- DOI: 10.1089/biores.2014.0059
Subcutaneous Adipose Tissue-Derived Stem Cell Utility Is Independent of Anatomical Harvest Site
Abstract
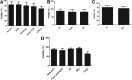
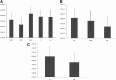
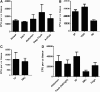
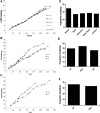
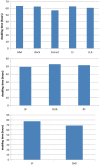
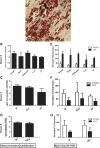
One of the challenges for tissue engineering and regenerative medicine is to obtain suitably large cell numbers for therapy. Mesenchymal stem cells (MSCs) can easily be expanded in vitro to obtain large numbers of cells, but this approach may induce cellular senescence. The characteristics of cells are dependent on variables like age, body mass index (BMI), and disease conditions, however, and in the case of adipose tissue-derived stem cells (ASCs), anatomical harvest site is also an important variable that can affect the regenerative potential of isolated cells. We therefore had kept the parameters (age, BMI, disease conditions) constant in this study to specifically assess influence of anatomical sites of individual donors on utility of ASCs. Adipose tissue was obtained from multiple anatomical sites in individual donors, and viability and nucleated cell yield were determined. MSC frequency was enumerated using colony forming unit assay and cells were characterized by flow cytometry. Growth characteristics were determined by long-term population doubling analysis of each sample. Finally, MSCs were induced to undergo adipogenic, osteogenic, and chondrogenic differentiation. To validate the findings, these results were compared with similar single harvest sites from multiple individual patients. The results of the current study indicated that MSCs obtained from multiple harvest sites in a single donor have similar morphology and phenotype. All adipose depots in a single donor exhibited similar MSC yield, viability, frequency, and growth characteristics. Equivalent differentiation capacity into osteocytes, adipocytes, and chondrocytes was also observed. On the basis of results, we conclude that it is acceptable to combine MSCs obtained from various anatomical locations in a single donor to obtain suitably large cell numbers required for therapy, avoiding in vitro senescence and lengthy and expensive in vitro culturing and expansion steps.
Keywords: mesenchymal stem cells; multiple harvest sites; regenerative potential.
Figures



References
-
- Dominici M, Le Blanc K, Mueller I, et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317 - PubMed
-
- Ringdén O, Uzunel M, Sundberg B, et al. . Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–2276 - PubMed
-
- Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525 - PubMed
-
- De Toni F, Poglio S, Youcef AB, et al. . Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev. 2011;20:2127–2138 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
