A Scalable Perfusion Culture System with Miniature Peristaltic Pumps for Live-Cell Imaging Assays with Provision for Microfabricated Scaffolds
- PMID: 26309810
- PMCID: PMC4534047
- DOI: 10.1089/biores.2015.0024
A Scalable Perfusion Culture System with Miniature Peristaltic Pumps for Live-Cell Imaging Assays with Provision for Microfabricated Scaffolds
Abstract
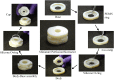
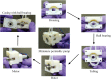
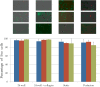
We present a perfusion culture system with miniature bioreactors and peristaltic pumps. The bioreactors are designed for perfusion, live-cell imaging studies, easy incorporation of microfabricated scaffolds, and convenience of operation in standard cell culture techniques. By combining with miniature peristaltic pumps-one for each bioreactor to avoid cross-contamination and to maintain desired flow rate in each-we have made a culture system that facilitates perfusion culture inside standard incubators. This scalable system can support multiple parallel perfusion experiments. The major components are fabricated by three-dimensional printing using VeroWhite, which we show to be amenable to ex vivo cell culture. Furthermore, the components of the system can be reused, thus making it economical. We validate the system and illustrate its versatility by culturing primary rat hepatocytes, live imaging the growth of mouse fibroblasts (NIH 3T3) on microfabricated ring-scaffolds inserted into the bioreactor, performing perfusion culture of breast cancer cells (MCF7), and high-magnification imaging of hepatocarcinoma cells (HuH7).
Keywords: 3D printing; VeroWhite; bioreactor; live imaging; microfabricated scaffolds; perfusion culture; peristaltic pump.
Figures








References
-
- Potter SM, Demarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110:17. - PubMed
-
- Hausen P, Riebesell M. A simple flow-through micro-chamber for handling fragile, small tissue explants and single non-adherent cells. Methods Cell Sci. 2003;168:165 - PubMed
-
- Tan G-DS, Toh GW, Birgersson E, et al. . A thin-walled polydimethylsiloxane bioreactor for high-density hepatocyte sandwich culture. Biotechnol Bioeng. 2013;110:1663. - PubMed
-
- Hing WA, Poole CA, Jensen CG, et al. . An integrated environmental perfusion chamber and heating system for long-term, high resolution imaging of living cells. J Microsc. 2000;199:90. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
