One health, multiple challenges: The inter-species transmission of influenza A virus
- PMID: 26309905
- PMCID: PMC4542011
- DOI: 10.1016/j.onehlt.2015.03.001
One health, multiple challenges: The inter-species transmission of influenza A virus
Abstract
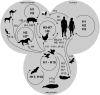
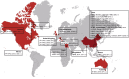
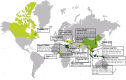
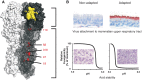
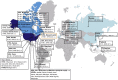
Influenza A viruses are amongst the most challenging viruses that threaten both human and animal health. Influenza A viruses are unique in many ways. Firstly, they are unique in the diversity of host species that they infect. This includes waterfowl (the original reservoir), terrestrial and aquatic poultry, swine, humans, horses, dog, cats, whales, seals and several other mammalian species. Secondly, they are unique in their capacity to evolve and adapt, following crossing the species barrier, in order to replicate and spread to other individuals within the new species. Finally, they are unique in the frequency of inter-species transmission events that occur. Indeed, the consequences of novel influenza virus strain in an immunologically naïve population can be devastating. The problems that influenza A viruses present for human and animal health are numerous. For example, influenza A viruses in humans represent a major economic and disease burden, whilst the poultry industry has suffered colossal damage due to repeated outbreaks of highly pathogenic avian influenza viruses. This review aims to provide a comprehensive overview of influenza A viruses by shedding light on interspecies virus transmission and summarising the current knowledge regarding how influenza viruses can adapt to a new host.
Figures

References
-
- Health WOfA Update on Highly Pathogenic Avian Influenza in Animals (TYPE H5 and H7) 2015. http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/... [cited; Available from:
-
- Cullinane A., Newton J. Equine influenza—a global perspective. Vet. Microbiol. 2013;167(1):205–214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
