Effect of Berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters
- PMID: 26310319
- PMCID: PMC4549888
- DOI: 10.1186/s12967-015-0629-3
Effect of Berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters
Abstract
Background: Berberine (BBR), as a new medicine for hyperlipidemia, can reduce the blood lipids in patients. Mechanistic studies have shown that BBR activates the extracellular-signal regulated kinase pathway by stabilizing low-density-lipoprotein receptor mRNA. However, aside from inhibiting the intestinal absorption of cholesterol, the effects of BBR on other metabolic pathways of cholesterol have not been reported. This study aimed to investigate the action of BBR on the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters.
Methods: Golden hamsters were fed a high-fat diet (HFD) for 6 weeks to induce hyperlipidemia, followed by oral treatment with 50 and 100 mg/kg/day of BBR or 10 and 30 mg/kg/day of lovastatin for 10 days, respectively. The levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), transaminases, and total bile acid in the serum, liver, bile and feces were measured using an enzyme-linked immunosorbent assay. The cholesterol (as well as coprostanol) levels in the liver, bile and feces were determined by gas chromatography-mass spectrometry.
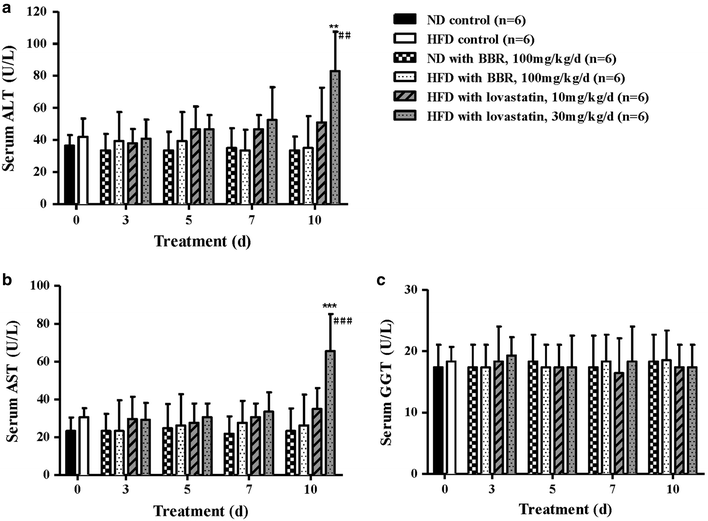
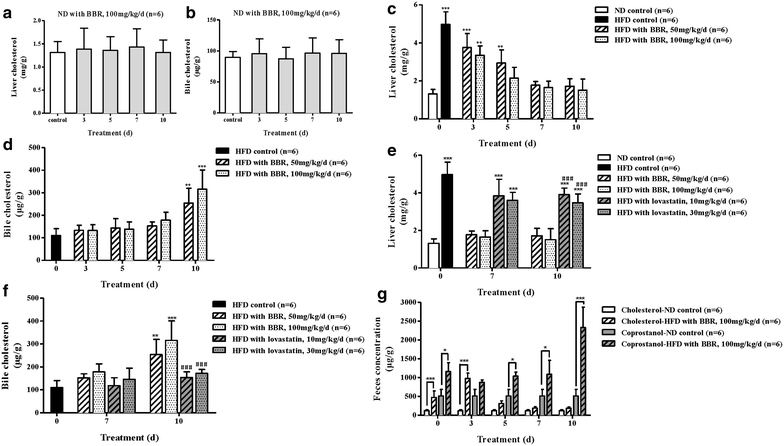
Results: The HFD hamsters showed significantly hyperlipidemic characteristics compared with the normal hamsters. Treatment with BBR for 10 days reduced the serum TC, TG and LDL-C levels in HFD hamsters by 44-70, 34-51 and 47-71%, respectively, and this effect was both dose- and time-dependent. Initially, a large amount of cholesterol accumulated in the hyperlipidemic hamster livers. After BBR treatment, reductions in the liver cholesterol were observed by day 3 and became significant by day 7 at both doses (P < 0.001). Meanwhile, bile cholesterol was elevated by day 3 and significantly increased at day 10 (P < 0.001). BBR promoted cholesterol excretion from the liver into the bile in hyperlipidemic hamsters but not in normal hamsters, and these results provide a link between the cholesterol-lowering effect of BBR with cholesterol excretion into the bile.
Conclusions: We conclude that BBR significantly promoted the excretion of cholesterol from the liver to the bile in hyperlipidemic hamsters, which led to large decreases in the serum TC, TG and LDL-C levels. Additionally, compared with lovastatin, the BBR treatment produced no obvious side effects on the liver function.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

